4-Methylumbelliferyl glucuronide contributes to hyaluronan synthesis inhibition
- PMID: 30914479
- PMCID: PMC6514619
- DOI: 10.1074/jbc.RA118.006166
4-Methylumbelliferyl glucuronide contributes to hyaluronan synthesis inhibition
Abstract
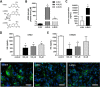
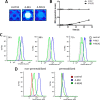
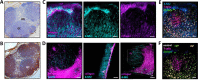
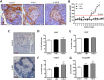
4-Methylumbelliferone (4-MU) inhibits hyaluronan (HA) synthesis and is an approved drug used for managing biliary spasm. However, rapid and efficient glucuronidation is thought to limit its utility for systemically inhibiting HA synthesis. In particular, 4-MU in mice has a short half-life, causing most of the drug to be present as the metabolite 4-methylumbelliferyl glucuronide (4-MUG), which makes it remarkable that 4-MU is effective at all. We report here that 4-MUG contributes to HA synthesis inhibition. We observed that oral administration of 4-MUG to mice inhibits HA synthesis, promotes FoxP3+ regulatory T-cell expansion, and prevents autoimmune diabetes. Mice fed either 4-MUG or 4-MU had equivalent 4-MU:4-MUG ratios in serum, liver, and pancreas, indicating that 4-MU and 4-MUG reach an equilibrium in these tissues. LC-tandem MS experiments revealed that 4-MUG is hydrolyzed to 4-MU in serum, thereby greatly increasing the effective bioavailability of 4-MU. Moreover, using intravital 2-photon microscopy, we found that 4-MUG (a nonfluorescent molecule) undergoes conversion into 4-MU (a fluorescent molecule) and that 4-MU is extensively tissue bound in the liver, fat, muscle, and pancreas of treated mice. 4-MUG also suppressed HA synthesis independently of its conversion into 4-MU and without depletion of the HA precursor UDP-glucuronic acid (GlcUA). Together, these results indicate that 4-MUG both directly and indirectly inhibits HA synthesis and that the effective bioavailability of 4-MU is higher than previously thought. These findings greatly alter the experimental and therapeutic possibilities for HA synthesis inhibition.
Keywords: 2-photon imaging; 4-methylumbelliferone; 4-methylumbelliferyl glucuronide; N-acetylglucosamine (GlcNAc); autoimmune disease; diabetes; extracellular matrix; glycosaminoglycan; hyaluronan; immunology.
© 2019 Nagy et al.
Conflict of interest statement
N.N., J. R., and P. L. B. are listed as inventors of the patents-pending (PCT/US2014/050770, S17-131US/BLSU-1-65422) filed by the Board of Trustees of the Leland Stanford Junior University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

