Rapid Replacement of Acinetobacter baumannii Strains Accompanied by Changes in Lipooligosaccharide Loci and Resistance Gene Repertoire
- PMID: 30914511
- PMCID: PMC6437055
- DOI: 10.1128/mBio.00356-19
Rapid Replacement of Acinetobacter baumannii Strains Accompanied by Changes in Lipooligosaccharide Loci and Resistance Gene Repertoire
Abstract
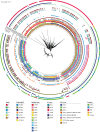
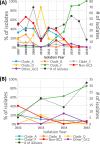
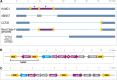
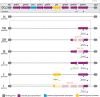
The population structure of health care-associated pathogens reflects patterns of diversification, selection, and dispersal over time. Empirical data detailing the long-term population dynamics of nosocomial pathogens provide information about how pathogens adapt in the face of exposure to diverse antimicrobial agents and other host and environmental pressures and can inform infection control priorities. Extensive sequencing of clinical isolates from one hospital spanning a decade and a second hospital in the Cleveland, OH, metropolitan area over a 3-year time period provided high-resolution genomic analysis of the Acinetobacter baumannii metapopulation. Genomic analysis demonstrated an almost complete replacement of the predominant strain groups with a new, genetically distinct strain group during the study period. The new group, termed clade F, differs from other global clone 2 (GC2) strains of A. baumannii in several ways, including its antibiotic resistance and lipooligosaccharide biosynthesis genes. Clade F strains are part of a large phylogenetic group with broad geographic representation. Phylogenetic analysis of single-nucleotide variants in core genome regions showed that although the Cleveland strains are phylogenetically distinct from those isolated from other locations, extensive intermixing of strains from the two hospital systems was apparent, suggesting either substantial exchange of strains or a shared, but geographically restricted, external pool from which infectious isolates were drawn. These findings document the rapid evolution of A. baumannii strains in two hospitals, with replacement of the predominant clade by a new clade with altered lipooligosaccharide loci and resistance gene repertoires.IMPORTANCE Multidrug-resistant (MDR) A. baumannii is a difficult-to-treat health care-associated pathogen. Knowing the resistance genes present in isolates causing infection aids in empirical treatment selection. Furthermore, knowledge of the genetic background can assist in tracking patterns of transmission to limit the spread of infections in hospitals. The appearance of a new genetic background in A. baumannii strains with a different set of resistance genes and cell surface structures suggests that strong selective pressures exist, even in highly MDR pathogens. Because the new strains have levels of antimicrobial resistance similar to those of the strains that were displaced, we hypothesize that other features, including host colonization and infection, may confer additional selective advantages and contribute to their increased prevalence.
Keywords: Acinetobacter; antibiotic resistance; genome analysis.
Copyright © 2019 Adams et al.
Figures
References
-
- Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, Galardini M, Nibbering PH, Earl AM, Ward DV, Paterson DL, Seifert H, Dijkshoorn L. 2012. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS One 7:e46984. doi: 10.1371/journal.pone.0046984. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

