Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer
- PMID: 30914635
- PMCID: PMC6435685
- DOI: 10.1038/s41467-019-09068-2
Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer
Abstract
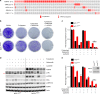
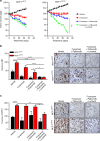
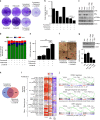
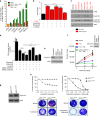
Using an ORF kinome screen in MCF-7 cells treated with the CDK4/6 inhibitor ribociclib plus fulvestrant, we identified FGFR1 as a mechanism of drug resistance. FGFR1-amplified/ER+ breast cancer cells and MCF-7 cells transduced with FGFR1 were resistant to fulvestrant ± ribociclib or palbociclib. This resistance was abrogated by treatment with the FGFR tyrosine kinase inhibitor (TKI) lucitanib. Addition of the FGFR TKI erdafitinib to palbociclib/fulvestrant induced complete responses of FGFR1-amplified/ER+ patient-derived-xenografts. Next generation sequencing of circulating tumor DNA (ctDNA) in 34 patients after progression on CDK4/6 inhibitors identified FGFR1/2 amplification or activating mutations in 14/34 (41%) post-progression specimens. Finally, ctDNA from patients enrolled in MONALEESA-2, the registration trial of ribociclib, showed that patients with FGFR1 amplification exhibited a shorter progression-free survival compared to patients with wild type FGFR1. Thus, we propose breast cancers with FGFR pathway alterations should be considered for trials using combinations of ER, CDK4/6 and FGFR antagonists.
Conflict of interest statement
C.L.A. receives grant support from Pfizer, Lilly, Bayer, and Radius. He serves in advisory roles to Symphogen, Daiichi Sankyo, TAIHO Oncology, Novartis, Merck, PUMA Biotechnology, Lilly, Radius, Sanofi, OrigiMed, and H3Biomedicine. He serves in the Scientific Advisory Board of the Komen Foundation. He holds stock options in Provista and Y-TRAP. R.J.N and R.B.L. are employees of Guardant Health; N.S., M.M., and F.S. are employees of Novartis Pharmaceuticals Corporation. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

