Vestibular processing during natural self-motion: implications for perception and action
- PMID: 30914780
- PMCID: PMC6611162
- DOI: 10.1038/s41583-019-0153-1
Vestibular processing during natural self-motion: implications for perception and action
Abstract
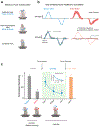
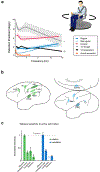
How the brain computes accurate estimates of our self-motion relative to the world and our orientation relative to gravity in order to ensure accurate perception and motor control is a fundamental neuroscientific question. Recent experiments have revealed that the vestibular system encodes this information during everyday activities using pathway-specific neural representations. Furthermore, new findings have established that vestibular signals are selectively combined with extravestibular information at the earliest stages of central vestibular processing in a manner that depends on the current behavioural goal. These findings have important implications for our understanding of the brain mechanisms that ensure accurate perception and behaviour during everyday activities and for our understanding of disorders of vestibular processing.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
-
- Curthoys IS & Halmagyi GM Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res 5, 67–107 (1995). - PubMed
-
- Peterka RJ, Wall C & Kentala E Determining the effectiveness of a vibrotactile balance prosthesis. J Vestib Res 16, 45–56 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

