Monocyte HLA-DR Assessment by a Novel Point-of-Care Device Is Feasible for Early Identification of ICU Patients With Complicated Courses-A Proof-of-Principle Study
- PMID: 30915080
- PMCID: PMC6423155
- DOI: 10.3389/fimmu.2019.00432
Monocyte HLA-DR Assessment by a Novel Point-of-Care Device Is Feasible for Early Identification of ICU Patients With Complicated Courses-A Proof-of-Principle Study
Abstract
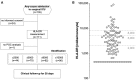
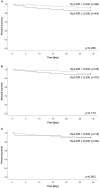
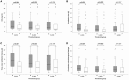
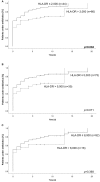
Background: Critically ill patients, especially following trauma or extensive surgery, experience a systemic immune response, consisting of a pro-inflammatory as well as a counterbalancing anti-inflammatory response. Pro-inflammation is necessary for the initiation of homeostatic control and wound healing of the organism. However, when the counterbalancing mechanisms dominate, a condition of secondary immunodeficiency occurs, which renders the patient susceptible for opportunistic or secondary infections. However, the incidence of this condition is yet illusive. Methods: For a period of 3 months (May to July 2017), 110 consecutive patients admitted to the surgical ICU of the Heidelberg University Hospital, a tertiary university hospital, were enrolled in the study. Monocyte HLA-DR (mHLA-DR), a long-known surrogate of monocyte function, was assessed quantitatively once on admission utilizing a novel point-of-care flow cytometer with single-use cartridges (Accelix system). Patients were followed up for further 28 days and data on ICU stay, antibiotic therapy, microbiological findings, and mechanical ventilation were recorded. Statistical analysis was performed to evaluate the incidence of immunosuppression-defined by different thresholds-as well as its consequence in terms of outcome and clinical course. Results: Depending on the HLA-DR threshold applied for stratification (≤8,000/≤5,000/≤2,000 molecules/cell), a large group of patients (85.5/68.2/40.0%) already presented with a robust decrease of HLA-DR on admission, independent of the cause for critical illness. Analyzed for survival, neither threshold was able to stratify patients with a higher mortality. However, both thresholds of 2,000 and 5,000 were able to discriminate patients with longer ICU stay, ventilation time and duration of antibiotic therapy, as well as higher count of microbiological findings. Moreover, a mHLA-DR value ≤2,000 molecules/cell was associated with higher incidence of overall antibiotic therapy. Conclusion: Single assessment of mHLA-DR using a novel point-of-care flow cytometer is able to stratify patients according to their risk of a complicated course. Therefore, this device overcomes the technical boundaries for measuring cellular biomarkers and paves the way for future studies involving personalized immunotherapy to patients with a high immunological risk profile independent of their background. Trial Registration: German Clinical Trials Register; ID: DRKS00012348.
Keywords: CARS; SIRS; immunosuppression; infection; personalized medicine; precision medicine; sepsis; tolerance.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

