A randomised phase II trial to examine feasibility of standardised, early palliative (STEP) care for patients with advanced cancer and their families [ACTRN12617000534381]: a research protocol
- PMID: 30915228
- PMCID: PMC6417202
- DOI: 10.1186/s40814-019-0424-7
A randomised phase II trial to examine feasibility of standardised, early palliative (STEP) care for patients with advanced cancer and their families [ACTRN12617000534381]: a research protocol
Abstract
Background: Current international consensus is that 'early' referral to palliative care services improves cancer patient and family carer outcomes; however, in practice, these referrals are not routine. Uncertainty about the 'best time' to refer has been highlighted as contributing to care variation. Previous work has identified clear disease-specific transition points in the cancer illness which heralded subsequent poor prognosis (less than 6 months) and which, we contest, represent times when palliative care should be routinely introduced as a standardised approach, if not already in place, to maximise patient and carer benefit. This protocol details a trial that will test the feasibility of a novel standardised outpatient model of early palliative care [Standardised Early Palliative Care (STEP Care)] for advanced cancer patients and their family carers, with referrals occurring at the defined disease-specific evidence-based transition points.The aims of this study are to (1) determine the feasibility of conducting a definitive phase 3 randomised trial, which evaluates effectiveness of STEP Care (compared to usual best practice cancer care) for patients with advanced breast or prostate cancer or high grade glioma; (2) examine preliminary efficacy of STEP Care on patient/family caregiver outcomes, including quality of life, mood, symptoms, illness understanding and overall survival; (3) document the impact of STEP Care on quality of end-of-life care; and (4) evaluate the timing of palliative care introduction according to patients, families and health care professionals.
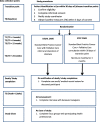
Methods: Phase 2, multicenter, open-label, parallel-arm, randomised controlled trial (RCT) of STEP Care plus standard best practice cancer care versus standard best practice cancer care alone.
Discussion: The research will test the feasibility of standardised palliative care introduction based on illness transitions and provide guidance on subsequent development of phase 3 studies of integration. This will directly address the current uncertainty about palliative care timing.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12617000534381.
Keywords: Family caregivers; Integrated care; Intervention; Palliative care; Quality of life; Trial; Unmet need.
Conflict of interest statement
Central ethical approval for the trial conduct at all participating sites was provided by the Human Research Ethics Committee at St Vincent’s Hospital Melbourne [HREC 179/16].Not applicableThe authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
LinkOut - more resources
Full Text Sources