Clinical impact of [18F]flutemetamol PET among memory clinic patients with an unclear diagnosis
- PMID: 30915522
- PMCID: PMC6486908
- DOI: 10.1007/s00259-019-04297-5
Clinical impact of [18F]flutemetamol PET among memory clinic patients with an unclear diagnosis
Abstract
Purpose: To investigate the impact of amyloid PET with [18F]flutemetamol on diagnosis and treatment management in a cohort of patients attending a tertiary memory clinic in whom, despite extensive cognitive assessment including neuropsychological testing, structural imaging, CSF biomarker analysis and in some cases [18F]FDG PET, the diagnosis remained unclear.
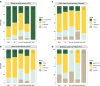
Methods: The study population consisted of 207 patients with a clinical diagnosis prior to [18F]flutemetamol PET including mild cognitive impairment (MCI; n = 131), Alzheimer's disease (AD; n = 41), non-AD (n = 10), dementia not otherwise specified (dementia NOS; n = 20) and subjective cognitive decline (SCD; n = 5).
Results: Amyloid positivity was found in 53% of MCI, 68% of AD, 20% of non-AD, 20% of dementia NOS, and 60% of SCD patients. [18F]Flutemetamol PET led, overall, to a change in diagnosis in 92 of the 207 patients (44%). A high percentage of patients with a change in diagnosis was observed in the MCI group (n = 67, 51%) and in the dementia NOS group (n = 11; 55%), followed by the non-AD and AD (30% and 20%, respectively). A significant increase in cholinesterase inhibitor treatment was observed after [18F]flutemetamol PET (+218%, 34 patients before and 108 patients after).
Conclusion: The present study lends support to the clinical value of amyloid PET in patients with an uncertain diagnosis in the tertiary memory clinic setting.
Keywords: Alzheimer’s disease; Amyloid PET; Cholinesterase inhibitors; Diagnostic change; [18F]Flutemetamol.
Conflict of interest statement
Conflicts of interest
J.L. is an employee of Hermes Medical Solutions, Stockholm, Sweden. All other authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures


References
-
- Nobili F, Arbizu J, Bouwman F, Drzezga A, Agosta F, Nestor P, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain (18)F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25:1201–1217. doi: 10.1111/ene.13728. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

