The molecular mechanism of dsRNA processing by a bacterial Dicer
- PMID: 30916338
- PMCID: PMC6511835
- DOI: 10.1093/nar/gkz208
The molecular mechanism of dsRNA processing by a bacterial Dicer
Abstract
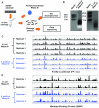
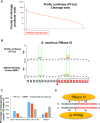
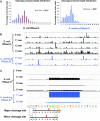
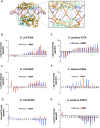
Members of the ribonuclease (RNase) III family regulate gene expression by processing dsRNAs. It was previously shown that Escherichia coli (Ec) RNase III recognizes dsRNA with little sequence specificity and the cleavage products are mainly 11 nucleotides (nt) long. It was also shown that the mutation of a glutamate (EcE38) to an alanine promotes generation of siRNA-like products typically 22 nt long. To fully characterize substrate specificity and product size of RNase IIIs, we performed in vitro cleavage of dsRNAs by Ec and Aquifex aeolicus (Aa) enzymes and delineated their products by next-generation sequencing. Surprisingly, we found that both enzymes cleave dsRNA at preferred sites, among which a guanine nucleotide was enriched at a specific position (+3G). Based on sequence and structure analyses, we conclude that RNase IIIs recognize +3G via a conserved glutamine (EcQ165/AaQ161) side chain. Abolishing this interaction by mutating the glutamine to an alanine eliminates the observed +3G preference. Furthermore, we identified a second glutamate (EcE65/AaE64), which, when mutated to alanine, also enhances the production of siRNA-like products. Based on these findings, we created a bacterial Dicer that is ideally suited for producing heterogeneous siRNA cocktails to be used in gene silencing studies.
Published by Oxford University Press on behalf of Nucleic Acids Research 2019.
Figures


References
-
- Robertson H.D., Webster R.E., Zinder N.D.. Purification and properties of ribonuclease III from Escherichia coli. J. Biol. Chem. 1968; 243:82–91. - PubMed
-
- Court D.L. Belasco JG, Brawerman G. Control of Messenger RNA Stability. 1993; NY: Academic Press; 71–116.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

