The RecQ helicase Sgs1 drives ATP-dependent disruption of Rad51 filaments
- PMID: 30916344
- PMCID: PMC6511845
- DOI: 10.1093/nar/gkz186
The RecQ helicase Sgs1 drives ATP-dependent disruption of Rad51 filaments
Erratum in
-
Correction.Nucleic Acids Res. 2024 Jun 10;52(10):6093. doi: 10.1093/nar/gkae352. Nucleic Acids Res. 2024. PMID: 38686812 Free PMC article. No abstract available.
Abstract
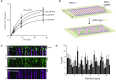
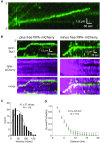
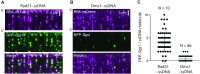
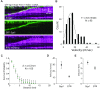
DNA helicases of the RecQ family are conserved among the three domains of life and play essential roles in genome maintenance. Mutations in several human RecQ helicases lead to diseases that are marked by cancer predisposition. The Saccharomyces cerevisiae RecQ helicase Sgs1 is orthologous to human BLM, defects in which cause the cancer-prone Bloom's Syndrome. Here, we use single-molecule imaging to provide a quantitative mechanistic understanding of Sgs1 activities on single stranded DNA (ssDNA), which is a central intermediate in all aspects of DNA metabolism. We show that Sgs1 acts upon ssDNA bound by either replication protein A (RPA) or the recombinase Rad51. Surprisingly, we find that Sgs1 utilizes a novel motor mechanism for disrupting ssDNA intermediates bound by the recombinase protein Rad51. The ability of Sgs1 to disrupt Rad51-ssDNA filaments may explain some of the defects engendered by RECQ helicase deficiencies in human cells.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
RECQ-like helicases Sgs1 and BLM regulate R-loop-associated genome instability.J Cell Biol. 2017 Dec 4;216(12):3991-4005. doi: 10.1083/jcb.201703168. Epub 2017 Oct 17. J Cell Biol. 2017. PMID: 29042409 Free PMC article.
-
Novel pro- and anti-recombination activities of the Bloom's syndrome helicase.Genes Dev. 2007 Dec 1;21(23):3085-94. doi: 10.1101/gad.1609007. Epub 2007 Nov 14. Genes Dev. 2007. PMID: 18003860 Free PMC article.
-
Sgs1 truncations induce genome rearrangements but suppress detrimental effects of BLM overexpression in Saccharomyces cerevisiae.J Mol Biol. 2011 Jan 28;405(4):877-91. doi: 10.1016/j.jmb.2010.11.035. Epub 2010 Nov 25. J Mol Biol. 2011. PMID: 21111748 Free PMC article.
-
[Functional analysis of yeast homologue gene associated with human DNA helicase causative syndromes].Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2002;(120):53-74. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2002. PMID: 12638184 Review. Japanese.
-
Maintenance of Yeast Genome Integrity by RecQ Family DNA Helicases.Genes (Basel). 2020 Feb 18;11(2):205. doi: 10.3390/genes11020205. Genes (Basel). 2020. PMID: 32085395 Free PMC article. Review.
Cited by
-
Replication protein A plays multifaceted roles complementary to specialized helicases in processing G-quadruplex DNA.iScience. 2021 May 1;24(5):102493. doi: 10.1016/j.isci.2021.102493. eCollection 2021 May 21. iScience. 2021. PMID: 34113828 Free PMC article.
-
The Regulation of Homologous Recombination by Helicases.Genes (Basel). 2020 May 1;11(5):498. doi: 10.3390/genes11050498. Genes (Basel). 2020. PMID: 32369918 Free PMC article. Review.
-
Hi-C sequencing unravels dynamic three-dimensional chromatin interactions in muntjac lineage: insights from chromosome fusions in Fea's muntjac genome.Chromosome Res. 2023 Nov 29;31(4):34. doi: 10.1007/s10577-023-09744-6. Chromosome Res. 2023. PMID: 38017297
-
A RAD51-ADP double filament structure unveils the mechanism of filament dynamics in homologous recombination.Nat Commun. 2023 Aug 17;14(1):4993. doi: 10.1038/s41467-023-40672-5. Nat Commun. 2023. PMID: 37591853 Free PMC article.
-
Single-molecule visualization of human BLM helicase as it acts upon double- and single-stranded DNA substrates.Nucleic Acids Res. 2019 Dec 2;47(21):11225-11237. doi: 10.1093/nar/gkz810. Nucleic Acids Res. 2019. PMID: 31544923 Free PMC article.
References
-
- Branzei D., Szakal B.. Building up and breaking down: mechanisms controlling recombination during replication. Crit. Rev. Biochem. Mol. Biol. 2017; 52:381–394. - PubMed
-
- Larsen N.B., Hickson I.D.. RecQ Helicases: conserved guardians of genomic integrity. Adv. Exp. Med. Biol. 2013; 767:161–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

