Mycobacterium tuberculosis infection of host cells in space and time
- PMID: 30916769
- PMCID: PMC6606852
- DOI: 10.1093/femsre/fuz006
Mycobacterium tuberculosis infection of host cells in space and time
Abstract
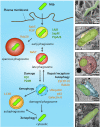
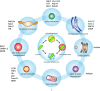
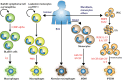
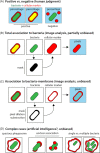
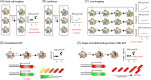
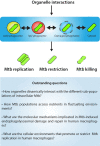
Tuberculosis (TB) caused by the bacterial pathogen Mycobacterium tuberculosis (Mtb) remains one of the deadliest infectious diseases with over a billion deaths in the past 200 years (Paulson 2013). TB causes more deaths worldwide than any other single infectious agent, with 10.4 million new cases and close to 1.7 million deaths in 2017. The obstacles that make TB hard to treat and eradicate are intrinsically linked to the intracellular lifestyle of Mtb. Mtb needs to replicate within human cells to disseminate to other individuals and cause disease. However, we still do not completely understand how Mtb manages to survive within eukaryotic cells and why some cells are able to eradicate this lethal pathogen. Here, we summarise the current knowledge of the complex host cell-pathogen interactions in TB and review the cellular mechanisms operating at the interface between Mtb and the human host cell, highlighting the technical and methodological challenges to investigating the cell biology of human host cell-Mtb interactions.
Keywords: Mycobacterium tuberculosis; Tuberculosis; autophagy; macrophage; phagosome.
© FEMS 2019.
Figures


References
-
- Anes E, Kuhnel MP, Bos Eet al. .. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol. 2003;5:793–802. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

