The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease
- PMID: 30916994
- PMCID: PMC6815102
- DOI: 10.1146/annurev-bioeng-060418-052139
The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease
Abstract
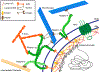
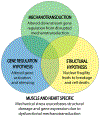
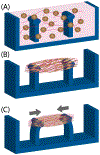
Cellular behavior is continuously affected by microenvironmental forces through the process of mechanotransduction, in which mechanical stimuli are rapidly converted to biochemical responses. Mounting evidence suggests that the nucleus itself is a mechanoresponsive element, reacting to cytoskeletal forces and mediating downstream biochemical responses. The nucleus responds through a host of mechanisms, including partial unfolding, conformational changes, and phosphorylation of nuclear envelope proteins; modulation of nuclear import/export; and altered chromatin organization, resulting in transcriptional changes. It is unclear which of these events present direct mechanotransduction processes and which are downstream of other mechanotransduction pathways. We critically review and discuss the current evidence for nuclear mechanotransduction, particularly in the context of stem cell fate, a largely unexplored topic, and in disease, where an improved understanding of nuclear mechanotransduction is beginning to open new treatment avenues. Finally, we discuss innovative technological developments that will allow outstanding questions in the rapidly growing field of nuclear mechanotransduction to be answered.
Keywords: LINC complex; lamin; laminopathies; mechanotransduction; nuclear mechanics; stem cells.
Figures


References
-
- Wang N, Tytell JD, Ingber DE. 2009. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol 10:75–82 - PubMed
-
- Wang N, Butler JP, Ingber DE. 1993. Mechanotransduction across the cell surface and through the cytoskleton. Science. 260:1124–27 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

