Telomerase Impinges on the Cellular Response to Oxidative Stress Through Mitochondrial ROS-Mediated Regulation of Autophagy
- PMID: 30917518
- PMCID: PMC6470917
- DOI: 10.3390/ijms20061509
Telomerase Impinges on the Cellular Response to Oxidative Stress Through Mitochondrial ROS-Mediated Regulation of Autophagy
Abstract
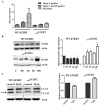
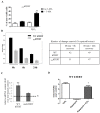
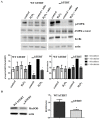
Telomerase has cellular functions beyond telomere stabilization, including a role in mitochondria. The function of the catalytic component-TERT-in mitochondria is still unknown, but it seems to play a role in the response to oxidative stress. Here, we interrogated the role of the subcellular localization of TERT to the response to hydrogen peroxide (H₂O₂) treatment. Using normal human fibroblasts (NHF) expressing non-tagged wild type (WT) human TERT (hTERT) or nuclear localization and function (nuchTERT), a mutant that we previously described as being competent in telomere elongation, while not being able to localize to mitochondria, we found the differential activation of autophagy as a function of hTERT's subcellular localization. Specifically, we found that only cells expressing the mutant had significant increases in autophagy markers as a response to H₂O₂ challenge. Either the reintroduction of the mitochondrial pool of hTERT or the expression of mitochondrially-targeted catalase in mutant cells blunted the autophagic response under oxidative stress. Interestingly, autophagy activation was also associated with decreased levels of mitochondrial DNA damage. Taken together, these results suggest that the loss of hTERT in mitochondria initiates a signaling cascade that allows for cells to adapt to and cope with the lack of mitochondrial telomerase. Such effects also influence the cellular response to oxidative damage.
Keywords: autophagy; mitochondria; oxidative stress; telomerase.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
-
- Listerman I., Sun J., Gazzaniga F.S., Lukas J.L., Blackburn E.H. The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. Cancer Res. 2013;73:2817–2828. doi: 10.1158/0008-5472.CAN-12-3082. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

