The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis
- PMID: 30917856
- PMCID: PMC6437920
- DOI: 10.1186/s13054-019-2395-8
The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis
Erratum in
-
Correction to: The effect of corticosteroids on the mortality of patients with influenza pneumonia: a systematic review and meta-analysis.Crit Care. 2020 Jun 23;24(1):376. doi: 10.1186/s13054-020-02996-2. Crit Care. 2020. PMID: 32576251 Free PMC article.
Abstract
Background: The effect of corticosteroids on clinical outcomes in patients with influenza pneumonia remains controversial. We aimed to further evaluate the influence of corticosteroids on mortality in adult patients with influenza pneumonia by comparing corticosteroid-treated and placebo-treated patients.
Methods: The PubMed, Embase, Medline, Cochrane Central Register of Controlled Trials (CENTRAL), and Information Sciences Institute (ISI) Web of Science databases were searched for all controlled studies that compared the effects of corticosteroids and placebo in adult patients with influenza pneumonia. The primary outcome was mortality, and the secondary outcomes were mechanical ventilation (MV) days, length of stay in the intensive care unit (ICU LOS), and the rate of secondary infection.
Results: Ten trials involving 6548 patients were pooled in our final analysis. Significant heterogeneity was found in all outcome measures except for ICU LOS (I2 = 38%, P = 0.21). Compared with placebo, corticosteroids were associated with higher mortality (risk ratio [RR] 1.75, 95% confidence interval [CI] 1.30 ~ 2.36, Z = 3.71, P = 0.0002), longer ICU LOS (mean difference [MD] 2.14, 95% CI 1.17 ~ 3.10, Z = 4.35, P < 0.0001), and a higher rate of secondary infection (RR 1.98, 95% CI 1.04 ~ 3.78, Z = 2.08, P = 0.04) but not MV days (MD 0.81, 95% CI - 1.23 ~ 2.84, Z = 0.78, P = 0.44) in patients with influenza pneumonia.
Conclusions: In patients with influenza pneumonia, corticosteroid use is associated with higher mortality.
Trial registration: PROSPERO (ID: CRD42018112384 ).
Keywords: Corticosteroids; Influenza pneumonia; Mortality.
Conflict of interest statement
Ethics approval and consent to participate
Each enrolled trial was approved by the corresponding institutional ethical committee, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





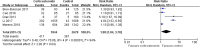
Comment in
-
Do Corticosteroids Benefit Patients With Influenza Pneumonia?Ann Emerg Med. 2020 Jan;75(1):100-101. doi: 10.1016/j.annemergmed.2019.06.021. Epub 2019 Jul 23. Ann Emerg Med. 2020. PMID: 31350095 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

