PNPLA2 influences secretion of triglyceride-rich lipoproteins by human hepatoma cells
- PMID: 30918066
- PMCID: PMC6547638
- DOI: 10.1194/jlr.M090928
PNPLA2 influences secretion of triglyceride-rich lipoproteins by human hepatoma cells
Abstract
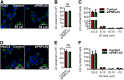
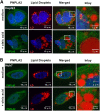
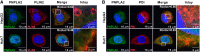
Patatin-like phospholipase domain-containing proteins (PNPLAs) are involved in triglyceride hydrolysis and lipid-droplet homeostasis in mice, but the physiological significance of the PNPLAs for triglyceride metabolism in human hepatocytes is unclear. Here, we investigate the roles of PNPLA2, PNPLA3, and PNPLA4 in triglyceride metabolism of human Huh7 and HepG2 hepatoma cells using gene-specific inhibition methods. siRNA inhibition of PNPLA3 or PNPLA4 is not associated with changes in triglyceride hydrolysis, secretion of triglyceride-rich lipoproteins (TRLs), or triglyceride accumulation. However, PNPLA2 siRNA inhibition, both in the absence and presence of oleate-containing medium, or treatment with the PNPLA2 inhibitor Atglistatin reduced intracellular triglyceride hydrolysis and decreased TRL secretion. In contrast, PNPLA2 inhibition showed no effects on lipid-droplet homeostasis, which is the primary physiological function of PNPLA2 in nonhepatic tissues. Moreover, confocal microscopy analysis found no clear evidence for the localization of PNPLA2 around lipid droplets. However, significant colocalization of PNPLA2 with the endoplasmic reticulum marker protein disulfide-isomerase was found in HepG2 and Huh7 cells with Rcoloc values of 0.61 ± 0.06 and 0.81 ± 0.05, respectively. In conclusion, PNPLA2 influences TRL secretion, but is not involved in lipid-droplet homeostasis in human hepatoma cells, a physiological role that is quite distinct from the metabolic function of PNPLA2 in nonhepatic tissues.
Keywords: confocal microscopy; endoplasmic reticulum; lipid droplets; lipolysis and fatty acid metabolism; liver; nonalcoholic fatty liver disease; triglycerides/diacylglycerol.
Copyright © 2019 Taxiarchis et al.
Figures




References
-
- Brunt E. M., Wong V. W., Nobili V., Day C. P., Sookoian S., Maher J. J., Bugianesi E., Sirlin C. B., Neuschwander-Tetri B. A., and Rinella R. E.. 2015. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers. 1: 15080. - PubMed
-
- Yang L. Y., Kuksis A., Myher J. J., and Steiner G.. 1995. Origin of triacylglycerol moiety of plasma very low density lipoproteins in the rat: structural studies. J. Lipid Res. 36: 125–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

