Identification of CP77 as the Third Orthopoxvirus SAMD9 and SAMD9L Inhibitor with Unique Specificity for a Rodent SAMD9L
- PMID: 30918078
- PMCID: PMC6613757
- DOI: 10.1128/JVI.00225-19
Identification of CP77 as the Third Orthopoxvirus SAMD9 and SAMD9L Inhibitor with Unique Specificity for a Rodent SAMD9L
Abstract
Orthopoxviruses (OPXVs) have a broad host range in mammalian cells, but Chinese hamster ovary (CHO) cells are nonpermissive for vaccinia virus (VACV). Here, we revealed a species-specific difference in host restriction factor SAMD9L as the cause for the restriction and identified orthopoxvirus CP77 as a unique inhibitor capable of antagonizing Chinese hamster SAMD9L (chSAMD9L). Two known VACV inhibitors of SAMD9 and SAMD9L (SAMD9&L), K1 and C7, can bind human and mouse SAMD9&L, but neither can bind chSAMD9L. Clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 knockout of chSAMD9L from CHO cells removed the restriction for VACV, while ectopic expression of chSAMD9L imposed the restriction for VACV in a human cell line, demonstrating that chSAMD9L is a potent restriction factor for VACV. In contrast to K1 and C7, cowpox virus CP77 can bind chSAMD9L and rescue VACV replication in cells expressing chSAMD9L, indicating that CP77 is yet another SAMD9L inhibitor but has a unique specificity for chSAMD9L. Binding studies showed that the N-terminal 382 amino acids of CP77 were sufficient for binding chSAMD9L and that both K1 and CP77 target a common internal region of SAMD9L. Growth studies with nearly all OPXV species showed that the ability of OPXVs to antagonize chSAMD9L correlates with CP77 gene status and that a functional CP77 ortholog was maintained in many OPXVs, including monkeypox virus. Our data suggest that a species-specific difference in rodent SAMD9L poses a barrier for cross-species OPXV infection and that OPXVs have evolved three SAMD9&L inhibitors with different specificities to overcome this barrier.IMPORTANCE Several OPXV species, including monkeypox virus and cowpox virus, cause zoonotic infection in humans. They are believed to use wild rodents as the reservoir or intermediate hosts, but the host or viral factors that are important for OPXV host range in rodents are unknown. Here, we showed that the abortive replication of several OPXV species in a Chinese hamster cell line was caused by a species-specific difference in the host antiviral factor SAMD9L, suggesting that SAMD9L divergence in different rodent species poses a barrier for cross-species OPXV infection. While the Chinese hamster SAMD9L could not be inhibited by two previously identified OPXV inhibitors of human and mouse SAMD9&L, it can be inhibited by cowpox virus CP77, indicating that OPXVs encode three SAMD9&L inhibitors with different specificities. Our data suggest that OPXV host range in broad rodent species depends on three SAMD9&L inhibitors with different specificities.
Keywords: host range; host restriction factor; poxvirus; vaccinia virus.
Copyright © 2019 American Society for Microbiology.
Figures


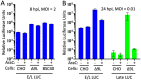
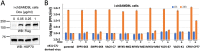




References
-
- Moss B. 2013. Poxviridae, p 2129–2159. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Damon IK. 2013. Poxviruses, p 2160–2184. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

