Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities
- PMID: 30918094
- PMCID: PMC6511080
- DOI: 10.1212/WNL.0000000000007370
Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities
Abstract
Objective: To identify distinct cognitive phenotypes in temporal lobe epilepsy (TLE) and evaluate patterns of white matter (WM) network alterations associated with each phenotype.
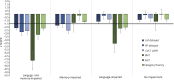
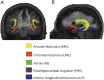
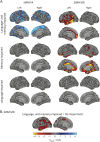
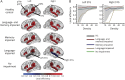
Methods: Seventy patients with TLE were characterized into 4 distinct cognitive phenotypes based on patterns of impairment in language and verbal memory measures (language and memory impaired, memory impaired only, language impaired only, no impairment). Diffusion tensor imaging was obtained in all patients and in 46 healthy controls (HC). Fractional anisotropy (FA) and mean diffusivity (MD) of the WM directly beneath neocortex (i.e., superficial WM [SWM]) and of deep WM tracts associated with memory and language were calculated for each phenotype. Regional and network-based SWM analyses were performed across phenotypes.
Results: The language and memory impaired group and the memory impaired group showed distinct patterns of microstructural abnormalities in SWM relative to HC. In addition, the language and memory impaired group showed widespread alterations in WM tracts and altered global SWM network topology. Patients with isolated language impairment exhibited poor network structure within perisylvian cortex, despite relatively intact global SWM network structure, whereas patients with no impairment appeared similar to HC across all measures.
Conclusions: These findings demonstrate a differential pattern of WM microstructural abnormalities across distinct cognitive phenotypes in TLE that can be appreciated at both the regional and network levels. These findings not only help to unravel the underlying neurobiology associated with cognitive impairment in TLE, but they could also aid in establishing cognitive taxonomies or in the prediction of cognitive course in TLE.
© 2019 American Academy of Neurology.
Figures

References
-
- Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain 2009;132:570–582. - PubMed
-
- Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 2003;54:425–432. - PubMed
-
- Hermann BP, Seidenberg M, Dow C, et al. . Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 2006;60:80–87. - PubMed
-
- Helmstaedter C, Hermann B, Lassonde M, Kahane P, Arzimanoglou A. Neuropsychology in the Care of People with Epilepsy. Montrouge, France: John Libbey Eurotext; 2011.
