Effect of dimethyl fumarate on lymphocytes in RRMS: Implications for clinical practice
- PMID: 30918100
- PMCID: PMC6511089
- DOI: 10.1212/WNL.0000000000007262
Effect of dimethyl fumarate on lymphocytes in RRMS: Implications for clinical practice
Abstract
Objective: To assess functional changes in lymphocyte repertoire and subsequent clinical implications during delayed-release dimethyl fumarate (DMF) treatment in patients with multiple sclerosis.
Methods: Using peripheral blood from several clinical trials of DMF, immune cell subsets were quantified using flow cytometry. For some patients, lymphocyte counts were assessed after DMF discontinuation. Incidence of adverse events, including serious and opportunistic infections, was assessed.
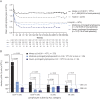
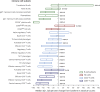
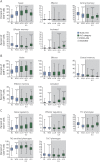
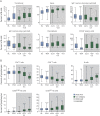
Results: In DMF-treated patients, absolute lymphocyte counts (ALCs) demonstrated a pattern of decline followed by stabilization, which also was reflected in the global reduction in numbers of circulating functional lymphocyte subsets. The relative frequencies of circulating memory T- and B-cell populations declined and naive cells increased. No increased incidence of serious infection or malignancy was observed for patients treated with DMF, even when stratified by ALC or T-cell subset frequencies. For patients who discontinued DMF due to lymphopenia, ALCs increased after DMF discontinuation; recovery time varied by ALC level at discontinuation. T-cell subsets closely correlated with ALCs in both longitudinal and cross-sectional analyses.
Conclusions: DMF shifted the immunophenotype of circulating lymphocyte subsets. ALCs were closely correlated with CD4+ and CD8+ T-cell counts, indicating that lymphocyte subset monitoring is not required for safety vigilance. No increased risk of serious infection was observed in patients with low T-cell subset counts. Monitoring ALC remains the most effective way of identifying patients at risk of subsequently developing prolonged moderate to severe lymphopenia, a risk factor for progressive multifocal leukoencephalopathy in DMF-treated patients.
Trial registration numbers: EUDRA CT 2015-001973-42, NCT00168701, NCT00420212, NCT00451451, and NCT00835770.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

Comment in
-
Dimethyl fumarate-induced changes in the MS lymphocyte repertoire: No need for subset monitoring.Neurology. 2019 Apr 9;92(15):696-697. doi: 10.1212/WNL.0000000000007255. Epub 2019 Mar 27. Neurology. 2019. PMID: 30918089 No abstract available.
References
-
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. - PubMed
-
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. - PubMed
-
- Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008;372:1463–1472. - PubMed
-
- Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med 2015;372:1476–1478. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
