SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea
- PMID: 30918101
- PMCID: PMC6511076
- DOI: 10.1212/WNL.0000000000007368
SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea
Abstract
Objective: This pivotal phase III study, SIAXI, investigated the efficacy and safety of incobotulinumtoxinA for the treatment of chronic sialorrhea due to Parkinson disease (PD), atypical parkinsonism, stroke, or traumatic brain injury (TBI).
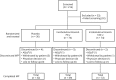
Methods: Adult patients with PD (70.7%), atypical parkinsonism (8.7%), stroke (19.0%), or TBI (2.7%) were randomized (2:2:1) to double-blind treatment with placebo (n = 36), or total doses of incobotulinumtoxinA 75 U (n = 74) or 100 U (n = 74), in a single treatment cycle. The coprimary endpoints were change in unstimulated salivary flow rate from baseline to week 4, and patients' Global Impression of Change Scale score at week 4. Adverse events were recorded throughout.
Results: A total of 184 patients were randomized. Both incobotulinumtoxinA dose groups showed reductions in mean unstimulated salivary flow rate at week 4, with a significant difference vs placebo in the incobotulinumtoxinA 100 U group (p = 0.004). Patients' Global Impression of Change Scale scores also improved at week 4, with a significant difference vs placebo in the incobotulinumtoxinA 100 U group (p = 0.002). A lasting effect was observed at week 16 post injection. The most frequent treatment-related adverse events in the incobotulinumtoxinA 75 U and 100 U groups were dry mouth (5.4% and 2.7% of patients) and dysphagia (2.7% and 0.0% of patients).
Conclusions: IncobotulinumtoxinA 100 U is an effective and well-tolerated treatment of chronic sialorrhea in adults.
Clinicaltrialsgov identifier: NCT02091739.
Classification of evidence: This study provides Class I evidence that incobotulinumtoxinA reduces salivary flow rates in patients with chronic sialorrhea due to PD, atypical parkinsonism, stroke, or TBI.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Parkes J, Hill N, Platt MJ, Donnelly C. Oromotor dysfunction and communication impairments in children with cerebral palsy: a register study. Dev Med Child Neurol 2010;52:1113–1119. - PubMed
-
- Kalf JG, Smit AM, Bloem BR, Zwarts MJ, Munneke M. Impact of drooling in Parkinson's disease. J Neurol 2007;254:1227–1232. - PubMed
-
- McGeachan AJ, Hobson EV, Shaw PJ, McDermott CJ. Developing an outcome measure for excessive saliva management in MND and an evaluation of saliva burden in Sheffield. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:108–113. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
