Clinical Correlates of Human Immunodeficiency Virus-1 (HIV-1) DNA and Inducible HIV-1 RNA Reservoirs in Peripheral Blood in Children With Perinatally Acquired HIV-1 Infection With Sustained Virologic Suppression for at Least 5 Years
- PMID: 30919879
- PMCID: PMC7319270
- DOI: 10.1093/cid/ciz251
Clinical Correlates of Human Immunodeficiency Virus-1 (HIV-1) DNA and Inducible HIV-1 RNA Reservoirs in Peripheral Blood in Children With Perinatally Acquired HIV-1 Infection With Sustained Virologic Suppression for at Least 5 Years
Abstract
Background: The Early Pediatric Initiation Canada Child Cure Cohort (EPIC4) study is a prospective, multicenter, Canadian cohort study investigating human immunodeficiency virus-1 (HIV-1) reservoirs, chronic inflammation, and immune responses in children with perinatally acquired HIV-1 infection. The focus of this report is HIV-1 reservoirs and correlates in the peripheral blood of children who achieved sustained virologic suppression (SVS) for ≥5 years.
Methods: HIV-1 reservoirs were determined by measuring HIV-1 DNA in peripheral blood mononuclear cells and inducible cell-free HIV-1 RNA in CD4+ T-cells by a prostratin analogue stimulation assay. HIV serology was quantified by signal-to-cutoff ratio (S/CO).
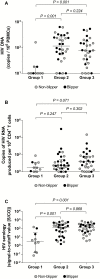
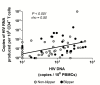
Results: Of 228 enrolled participants, 69 achieved SVS for ≥5 years. HIV-1 DNA, inducible cell-free HIV-1 RNA, and S/COs correlated directly with the age of effective combination antiretroviral therapy (cART) initiation (P < .001, P = .036, and P < .001, respectively) and age when SVS was achieved (P = .002, P = .038, and P < .001, respectively) and inversely with the proportion of life spent on effective cART (P < .001, P = .01, and P < .001, respectively) and proportion of life spent with SVS (P < .001, P = .079, and P < .001, respectively). Inducible cell-free HIV-1 RNA correlated with HIV-1 DNA, most particularly in children with SVS, without virologic blips, that was achieved with the first cART regimen initiated prior to 6 months of age (rho = 0.74; P = .037) or later (rho = 0.87; P < .001). S/COs correlated with HIV-1 DNA (P = .003), but less so with inducible cell-free HIV-1 RNA (P = .09).
Conclusions: The prostratin analogue stimulation assay, with its lower blood volume requirement, could be a valuable method for evaluating inducible HIV-1 reservoirs in children. Standard commercial HIV serology may be a practical initial indirect measure of reservoir size in the peripheral blood of children with perinatally acquired HIV-1 infection.
Keywords: HIV-1 DNA; child; inducible RNA; prostratin analogue; reservoir.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Strain MC, Little SJ, Daar ES, et al. . Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005; 191:1410–8. - PubMed
-
- Laanani M, Ghosn J, Essat A, et al. ; Agence Nationale de Recherche sur le Sida PRIMO Cohort Study Group Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60:1715–21. - PubMed
-
- Persaud D, Patel K, Karalius B, et al. ; Pediatric Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome Cohort Study Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168:1138–46. - PMC - PubMed
-
- Foster C, Pace M, Kaye S, et al. ; CHERUB Investigators Early antiretroviral therapy reduces HIV DNA following perinatal HIV infection. AIDS 2017; 31:1847–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

