Maize glossy6 is involved in cuticular wax deposition and drought tolerance
- PMID: 30919902
- PMCID: PMC6598097
- DOI: 10.1093/jxb/erz131
Maize glossy6 is involved in cuticular wax deposition and drought tolerance
Abstract
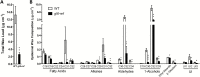
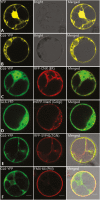
Cuticular waxes, long-chain hydrocarbon compounds, form the outermost layer of plant surfaces in most terrestrial plants. The presence of cuticular waxes protects plants from water loss and other environmental stresses. Cloning and characterization of genes involved in the regulation, biosynthesis, and extracellular transport of cuticular waxes onto the surface of epidermal cells have revealed the molecular basis of cuticular wax accumulation. However, intracellular trafficking of synthesized waxes to the plasma membrane for cellular secretion is poorly understood. Here, we characterized a maize glossy (gl6) mutant that exhibited decreased epicuticular wax load, increased cuticle permeability, and reduced seedling drought tolerance relative to wild-type. We combined an RNA-sequencing-based mapping approach (BSR-Seq) and chromosome walking to identify the gl6 candidate gene, which was confirmed via the analysis of multiple independent mutant alleles. The gl6 gene represents a novel maize glossy gene containing a conserved, but uncharacterized, DUF538 domain. This study suggests that the GL6 protein may be involved in the intracellular trafficking of cuticular waxes, opening the door to elucidating the poorly understood process by which cuticular wax is transported from its site of biosynthesis to the plasma membrane.
Keywords: glossy6 ( gl6 ); Cuticular waxes; DUF538; drought tolerance; glossy mutant; maize (Zea mays).
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




Similar articles
-
ZmEREB46, a maize ortholog of Arabidopsis WAX INDUCER1/SHINE1, is involved in the biosynthesis of leaf epicuticular very-long-chain waxes and drought tolerance.Plant Sci. 2022 Aug;321:111256. doi: 10.1016/j.plantsci.2022.111256. Epub 2022 May 18. Plant Sci. 2022. PMID: 35696901
-
OsGL1-3 is involved in cuticular wax biosynthesis and tolerance to water deficit in rice.PLoS One. 2015 Jan 2;10(1):e116676. doi: 10.1371/journal.pone.0116676. eCollection 2015. PLoS One. 2015. PMID: 25555239 Free PMC article.
-
Co-expression analysis aids in the identification of genes in the cuticular wax pathway in maize.Plant J. 2019 Feb;97(3):530-542. doi: 10.1111/tpj.14140. Epub 2018 Dec 7. Plant J. 2019. PMID: 30375131
-
MdCER2 conferred to wax accumulation and increased drought tolerance in plants.Plant Physiol Biochem. 2020 Apr;149:277-285. doi: 10.1016/j.plaphy.2020.02.013. Epub 2020 Feb 14. Plant Physiol Biochem. 2020. PMID: 32088579 Review.
-
Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species.Plant Cell Rep. 2015 Apr;34(4):557-72. doi: 10.1007/s00299-015-1772-2. Epub 2015 Feb 19. Plant Cell Rep. 2015. PMID: 25693495 Review.
Cited by
-
The FUSED LEAVES1-ADHERENT1 regulatory module is required for maize cuticle development and organ separation.New Phytol. 2021 Jan;229(1):388-402. doi: 10.1111/nph.16837. Epub 2020 Aug 27. New Phytol. 2021. PMID: 32738820 Free PMC article.
-
Optogenetic and Chemical Induction Systems for Regulation of Transgene Expression in Plants: Use in Basic and Applied Research.Int J Mol Sci. 2022 Feb 3;23(3):1737. doi: 10.3390/ijms23031737. Int J Mol Sci. 2022. PMID: 35163658 Free PMC article. Review.
-
Germination Development of Powdery Mildew on Natural and Artificial Wheat Leaf Surfaces: A Study to Investigate Plant Wax Signals.Small Sci. 2023 Jan 31;3(3):2200092. doi: 10.1002/smsc.202200092. eCollection 2023 Mar. Small Sci. 2023. PMID: 40212061 Free PMC article.
-
Drought Resistance by Engineering Plant Tissue-Specific Responses.Front Plant Sci. 2020 Jan 22;10:1676. doi: 10.3389/fpls.2019.01676. eCollection 2019. Front Plant Sci. 2020. PMID: 32038670 Free PMC article. Review.
-
Domain of unknown function (DUF) proteins in plants: function and perspective.Protoplasma. 2024 May;261(3):397-410. doi: 10.1007/s00709-023-01917-8. Epub 2023 Dec 30. Protoplasma. 2024. PMID: 38158398 Review.
References
-
- Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J. 2012. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. The Plant Cell 24, 3106–3118. - PMC - PubMed
-
- Bernard A, Joubès J. 2013. Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Progress in Lipid Research 52, 110–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

