Ab interno trabecular bypass surgery with iStent for open-angle glaucoma
- PMID: 30919929
- PMCID: PMC6437719
- DOI: 10.1002/14651858.CD012743.pub2
Ab interno trabecular bypass surgery with iStent for open-angle glaucoma
Abstract
Background: Glaucoma is a leading cause of irreversible blindness worldwide. In early stages, glaucoma results in progressive loss of peripheral (side) vision; in later stages, it results in loss of central vision leading to blindness. Elevated intraocular pressure (IOP) is the only known modifiable risk factor for glaucoma. Minimally invasive glaucoma surgical (MIGS) techniques, such as ab interno trabecular bypass surgery with iStent (Glaukos Corporation, Laguna Hills, CA, USA), have been introduced as a new treatment modality for glaucoma. However, the effectiveness of MIGS on keeping people 'drop-free' (i.e. not having to use eye drops to control IOP) and other outcomes is uncertain.
Objectives: To assess the effectiveness and safety of ab interno trabecular bypass surgery with iStent (or iStent inject) for open-angle glaucoma in comparison to conventional medical, laser, or surgical treatment.
Search methods: Cochrane Eyes and Vision's Information Specialist searched the following databases on 17 August 2018: the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register; 2018, Issue 7), MEDLINE Ovid, Embase Ovid, the ISRCTN registry, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We applied no date or language restrictions. We searched the reference lists of reports from included studies.
Selection criteria: We included randomized controlled trials (RCTs) that had compared iStent or iStent inject to medical therapy, laser treatment, conventional glaucoma surgery (trabeculectomy), or other MIGS procedures. We included RCTs that had compared iStent or iStent inject in combination with phacoemulsification to phacoemulsification alone.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Two review authors independently screened search results, assessed risk of bias, and extracted data from reports of included RCTs using an electronic data collection form.
Main results: We included seven RCTs (765 eyes of 764 participants; range per study 33 to 239 participants) that evaluated iStent in people with open-angle glaucoma. We also identified 13 studies that are ongoing or awaiting publications of results. Most participants in the included studies were women (417/764 (55%) participants) and older age (age range: 49 to 89 years). We assessed most trials at unclear or high risk of bias: four trials did not clearly report the method of generating the random sequence or concealing allocation; five were unmasked, open-label studies, which we assessed at high risk of bias for performance and detection bias. All seven trials were funded by the Glaukos Corporation. We graded the certainty of evidence as very low.Four RCTs compared iStent in combination with phacoemulsification to phacoemulsification alone. The summary estimate which we derived from two of the four RCTs suggested that participants in the iStent in combination with phacoemulsification group were 1.38 times more likely to be drop-free between six and 18 months than those in the phacoemulsification alone group (risk ratio (RR) 1.38, 95% confidence interval (CI) 1.18 to 1.63, I2 = 67%). Data from two RCTs also suggested that iStent in combination with phacoemulsification compared to phacoemulsification alone may have offered a small reduction in number of IOP-lowering drops (mean difference (MD) -0.42 drops, 95% CI -0.60 to -0.23). It is uncertain whether there was any difference in terms of mean reduction in IOP from baseline (no meta-analysis).Two RCTs compared treatment with iStent to medical therapy; one of the two trials used the iStent inject. We determined the two trials to be clinically and methodologically heterogeneous and did not conduct a meta-analysis; however, the investigators of both trials reported that over 90% of participants in the treatment groups were drop-free compared to no participants in the medical therapy groups at six to 18 months.One RCT compared treatment with one versus two versus three iStents. There was no difference in terms of participants who were drop-free at 36 months or less; however, at longer follow-up (i.e. at 42 months) participants in the one iStent treatment were less likely to be drop-free than those in the two iStent (RR 0.51, 95% CI 0.34 to 0.75) or three iStent (RR 0.49, 95% CI 0.34 to 0.73) treatment groups. The study did not report the mean change in number of IOP-lowering drops.The type and timing of complications reported varied by RCTs. Similar proportions of participants who underwent treatment with iStent in combination with phacoemulsification and who underwent phacoemulsification alone needed secondary glaucoma surgery. None of RCTs reported findings related to quality of life.
Authors' conclusions: There is very low-quality evidence that treatment with iStent may result in higher proportions of participants who are drop-free or achieving better IOP control, in the short, medium, or long-term. Results from the 13 studies with results not yet available may clarify the benefits of treatment of people with iStent. Additionally, future MIGS studies should consider measuring quality of life and outcomes that reflect people's ability to perform vision-dependent activities.
Conflict of interest statement
JL: This Cochrane Review was prepared while Dr. Le was a doctoral candidate at the Johns Hopkins Bloomberg School of Public Health. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. AB: none. LW: none. TL: none.
Figures
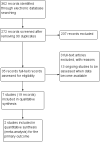






Update of
References
References to studies included in this review
Fea 2010 {published data only}
-
- Fea A, Lacroix G, Ghennadian S, Brogliatti B, Grignolo F. A prospective, randomized, double blind 15‐month pilot study on the efficacy of trabecular stent (iStent) implantation with combined phacoemulsification. American Academy of Ophthalmology 2008:214.
-
- Fea AM. Phacoemulsification versus phacoemulsification with micro‐bypass stent implantation in primary open‐angle glaucoma: randomized double‐masked clinical trial. Journal of Cataract and Refractive Surgery 2010;36(3):407‐12. - PubMed
-
- NCT00847158. A clinical trial of phacoemulsification versus phacoemulsification and the iStent implantation in POAG patients. clinicaltrials.gov/ct2/show/NCT00847158 (first received 9 February 2009).
Fea 2014 {published data only}
-
- NCT00913029. Evaluation of the iStent versus two ocular hypotensive agents in patients with primary open‐angle glaucoma (POAG) (SL). clinicaltrials.gov/ct2/show/study/NCT00913029 (first received 3 June 2009).
Fernandez‐Barrientos 2010 {published data only}
-
- Fernandez‐Barrientos Y, Garcia‐Feijoo J, Martinez‐de‐la‐Casa JM, Pablo LE, Fernandez‐Perez C, Garcia Sanchez J. Fluorophotometric study of the effect of the Glaukos trabecular microbypass stent on aqueous humor dynamics. Investigative Ophthalmology and Visual Science 2010;51(7):3327‐32. - PubMed
-
- NCT00326066. A study of the iStent in combo with cataract surgery in newly diagnosed open angle glaucoma or OH patients. clinicaltrials.gov/ct2/show/NCT00326066 (first received 13 May 2006).
Katz 2015 {published data only}
-
- Katz LJ, Erb C, Carceller Guillamet A, Fea AM, Voskanyan L, Giamporcaro JE, et al. Long‐term titrated IOP control with one, two, or three trabecular micro‐bypass stents in open‐angle glaucoma subjects on topical hypotensive medication: 42‐month outcomes. Clinical Ophthalmology 2018;12:255‐62. - PMC - PubMed
-
- NCT01517477. One, two, or three iStents for the reduction of intraocular pressure in open‐angle glaucoma subjects. clinicaltrials.gov/ct2/show/NCT01517477 (first received 25 January 2012).
NCT00721968 {unpublished data only}
-
- NCT00721968. Safety and efficacy of the GTS400 stent in conjunction with cataract. clinicaltrials.gov/ct2/show/NCT00721968 (first received 6 March 2014).
Samuelson 2011 {published data only}
-
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro‐bypass stent implantation in patients with mild‐to‐moderate open‐angle glaucoma and cataract: two‐year follow‐up. Journal of Cataract and Refractive Surgery 2012;38(8):1339‐45. - PubMed
-
- NCT00323284. A study of the trabecular micro‐bypass stent in combination with cataract surgery in subjects with open angle glaucoma. clinicaltrials.gov/ct2/show/NCT00323284 (first received 4 October 2012).
-
- Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro‐bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 2011;118(3):459‐67. - PubMed
Vold 2016 {published data only}
-
- NCT01443988. Subjects with open‐angle glaucoma, pseudoexfoliative glaucoma, or ocular hypertension naïve to medical and surgical therapy, treated with two trabecular micro‐bypass stents (iStent) or travoprost. clinicaltrials.gov/ct2/show/NCT01443988 (first received 30 September 2011).
References to studies excluded from this review
Bacharach 2014 {published data only}
-
- Bacharach J. Efficacy of trabecular bypass stent through 2 years: data from the United States pivotal trial. Journal of Cataract and Refractive Surgery 2014; Vol. 40, issue 8:1325‐6. - PubMed
NCT03274323 {published data only}
-
- NCT03274323. Trabeculectomy versus 2‐iStent and prostaglandin study (TIPS). clinicaltrials.gov/ct2/show/NCT03274323 (first received 6 September 2017).
Vlasov 2017 {published data only}
-
- Vlasov A, Kim WI. The efficacy of two trabecular bypass stents compared to one in the management of open‐angle glaucoma. Military Medicine 2017;182(S1):222‐5. - PubMed
References to ongoing studies
EUCTR2009‐018066‐36‐ES {published data only}
-
- EUCTR2009‐018066‐36‐ES. Open prospective randomized evaluation of iStent® (GTS400) versus two ocular hypotensive agents in patients with primary open‐angle glaucoma (Google translation) [Evaluación aleatorizada prospectiva en abierto del iStent® (GTS400) frente a dos agentes hipotensores oculares en pacientes con glaucoma primario de ángulo abierto – second line study]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐018066‐36‐ES (first received 11 March 2010).
NCT00326079 {published data only}
-
- NCT00326079. A study of the Glaukos trabecular micro‐bypass stent in open angle glaucoma subjects 1 stent versus 2. clinicaltrials.gov/ct2/show/study/NCT00326079 (first received 16 May 2016).
NCT01052558 {published data only}
-
- NCT01052558. GTS400 stent implantation in conjunction with cataract surgery in subjects with open‐angle glaucoma. clinicaltrials.gov/ct2/show/NCT01052558 (first received 20 January 2010).
NCT01252849 {published data only}
-
- NCT01252849. Purpose of this study is to evaluate the safety and efficacy of one, two, or three iStents for the reduction of intraocular pressure in open‐angle glaucoma subjects. clinicaltrials.gov/ct2/show/NCT01252849 (first received 3 December 2010).
NCT01444040 {published data only}
-
- NCT01444040. Subjects with open‐angle glaucoma, pseudoexfoliative glaucoma, or ocular hypertension naïve to medical and surgical therapy, treated with two trabecular micro‐bypass stents (iStent inject) or travoprost. clinicaltrials.gov/ct2/show/NCT01444040 (first received 30 September 2011).
NCT01444105 {published data only}
-
- NCT01444105. Open‐angle glaucoma subjects on one ocular hypotensive medication randomized to treatment with two trabecular micro‐bypass stents or selective laser trabeculoplasty. clinicaltrials.gov/ct2/show/NCT01444105 (first received 30 September 2011).
NCT01455467 {published data only}
-
- NCT01455467. Open‐angle glaucoma subjects on one topical hypotensive medication randomized to treatment with one or two trabecular micro‐bypass stents in conjunction with cataract surgery. clinicaltrials.gov/ct2/show/NCT01455467 (first received 20 October 2011).
NCT01461291 {published data only}
-
- NCT01461291. Multicenter study using Glaukos® trabecular micro‐bypass stent model GTS400 using the G2‐M‐IS injector system in conjunction with cataract surgery. clinicaltrials.gov/ct2/show/NCT01461291 (first received 28 October 2011).
NCT02023242 {published data only}
-
- NCT02023242. Comparing effectiveness of the Hydrus Microstent (TM) to two iStents to lower IOP in phakic eyes (COMPARE) [A prospective, multicenter, randomized comparison of the Hydrus to the iStent® for lowering intraocular pressure in primary open angle glaucoma]. clinicaltrials.gov/ct2/show/study/NCT02023242 (first received 30 December 2013).
NCT02024464 {published data only}
-
- NCT02024464. Comparing Hydrus Microstent(TM) to the iStent for lowering IOP in glaucoma patients undergoing cataract surgery. clinicaltrials.gov/ct2/show/NCT02024464 (first received 31 December 2013).
NCT02327312 {published data only}
-
- NCT02327312. Multicenter investigation of trabecular micro‐bypass stents vs. laser trabeculoplasty. clinicaltrials.gov/ct2/show/NCT02327312 (first received 30 December 2014).
NCT03106181 {published data only}
-
- NCT03106181. A comparison of cataract surgery alone and cataract surgery with iStent. clinicaltrials.gov/ct2/show/NCT03106181 (first received 10 April 2017).
UMIN000027734 {published data only}
-
- UMIN000027734. A randomized study of efficacy of trabecular micro bypass stent with cataract surgery for open angle glaucoma. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000031775 first received 6 June 2017.
Additional references
AAO 2015
-
- Prum BE Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary open‐angle glaucoma preferred practice pattern® guidelines. Ophthalmology 2016;123(1):P41‐P111. - PubMed
AGIS 2000
-
- AGIS investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. American Journal of Ophthalmology 2000;130(4):429‐40. - PubMed
Allingham 2010
-
- Allingham RR, Damji KF, Freedman SF, Moroi SE, Rhee DJ, Shields MB. Shields Textbook of Glaucoma. 6th Edition. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins, 2010.
Bahler 2012
-
- Bahler CK, Hann CR, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second‐generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. American Journal of Ophthalmology 2012;153(6):1206‐13. - PubMed
Brandao 2013
Caprioli 2015
-
- Caprioli J, Kim JH, Friedman DS, Kiang T, Moster MR, Parrish RK 2nd, et al. Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology 2015;122(9):1795‐801. - PubMed
Covidence 2015 [Computer program]
-
- Veritas Health Innovation. Covidence. Version accessed prior to 25 April 2017. Melbourne: Veritas Health Innovation, 2015.
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from handbook.cochrane.org.
EGS 2014
-
- European Glaucoma Society. Terminology and Guidelines for Glaucoma. 4th Edition. Savona: PubliComm, 2014.
Francis 2011
-
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011;118(7):1466‐80. - PubMed
Friedman 2009
-
- Friedman DS, Okeke CO, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology 2009;116(6):1097‐105. - PubMed
Gedde 2012a
-
- Gedde SJ, Singh K, Schiffman JC, Feuer WJ. The Tube Versus Trabeculectomy study: interpretation of results and application to clinical practice. Current Opinion in Ophthalmology 2012;23(2):118‐26. - PubMed
Gedde 2012b
Glanville 2006
Glaukos 2016
-
- Glaukos Corporation. The iStent procedure. www.glaukos.com/istent (accessed on 27 June 2016).
GRADEpro GDT 2015 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 14 January 2019. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence – publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. - PubMed
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hu 2016
King 2018
Kirwan 2013
-
- Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013;120(12):2532‐9. - PubMed
Klamann 2015
-
- Klamann MK, Gonnermann J, Pahlitzsch M, Maier AK, Joussen AM, Torun N, et al. iStent inject in phakic open angle glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology 2015;253(6):941‐7. - PubMed
Larsen 2017
Lavia 2017
Le 2016
Le 2018a
Le 2018b
-
- Le JT, Bicket, AK Tarver ME, Eydelman M, Li T. Identifying and prioritizing outcomes that matter to patients considering minimally invasive glaucoma surgeries (MIGS) and other treatments for open‐angle glaucoma. American Glaucoma Society Meeting; 2018 Mar 1‐4; New York City (NY). 2018.
Leahy 2015
Li 2016
Lichter 2001
-
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108(11):1943‐53. - PubMed
Malvankar‐Mehta 2015
Manasses 2016
Medeiros 2015
NEI 2015
-
- National Eye Institute. Glaucoma, open‐angle. nei.nih.gov/eyedata/glaucoma (accessed 27 October 2015).
NICE 2009
-
- National Institute for Health and Care Excellence. Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension. www.nice.org.uk/guidance/CG85/chapter/1‐Guidance#treatment‐for‐people‐wi... (accessed 5 June 2017).
Okeke 2009
-
- Okeke CO, Quigley HA, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Adherence with topical glaucoma medication monitored electronically. The Travatan Dosing Aid study. Ophthalmology 2009;116(2):191‐9. - PubMed
Otarola 2017
Patel 2015
Quigley 2006
Quigley 2007
-
- Quigley HA, Friedman DS, Hahn SR. Evaluation of practice patterns for the care of open‐angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology 2007;114(9):1599‐606. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rolim de Moura 2007
Spaeth 2015
Tarver 2017
-
- Tarver M, Eyldelman M. Incorporating patients' perspectives. glaucomatoday.com/2017/04/incorporating‐patients‐perspectives (accessed 15 April 2018).
Tham 2014
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology 2014;121(11):2081‐90. - PubMed
Tóth 2019
Woo 2015
-
- Woo DM, Healey PR, Graham SL, Goldberg I. Intraocular pressure‐lowering medications and long‐term outcomes of selective laser trabeculoplasty. Clinical and Experimental Ophthalmology 2015;43(4):320‐7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

