A phase III randomized, multicentre, double blind, active controlled trial to compare the efficacy and safety of two different anagrelide formulations in patients with essential thrombocythaemia - the TEAM-ET 2·0 trial
- PMID: 30919941
- PMCID: PMC6594023
- DOI: 10.1111/bjh.15824
A phase III randomized, multicentre, double blind, active controlled trial to compare the efficacy and safety of two different anagrelide formulations in patients with essential thrombocythaemia - the TEAM-ET 2·0 trial
Abstract
Anagrelide is an established treatment option for essential thrombocythaemia (ET). A prolonged release formulation was developed with the aim of reducing dosing frequency and improving tolerability, without diminishing efficacy. This multicentre, randomized, double blind, active-controlled, non-inferiority trial investigated the efficacy, safety and tolerability of anagrelide prolonged release (A-PR) over a reference product in high-risk ET patients, either anagrelide-naïve or -experienced. In a 6 to 12-week titration period the individual dose for the consecutive 4-week maintenance period was identified. The primary endpoint was the mean platelet count during the maintenance period (3 consecutive measurements, day 0, 14, 28). Of 112 included patients 106 were randomized. The mean screening platelet counts were 822 × 109 /l (95% confidence interval (CI) 707-936 × 109 /l) and 797 × 109 /l (95% CI 708-883 × 109 /l) for A-PR and the reference product, respectively. Both treatments effectively reduced platelet counts, to mean 281 × 109 /l for A-PR (95% CI 254-311) and 305 × 109 /l (95% CI 276-337) for the reference product (P < 0·0001, for non-inferiority). Safety and tolerability were comparable between both drugs. The novel prolonged-release formulation was equally effective and well tolerated compared to the reference product. A-PR provides a more convenient dosing schedule and will offer an alternative to licensed immediate-release anagrelide formulations.
Keywords: anagrelide; essential thrombocythaemia; pharmacodynamics; pharmacokinetics; safety.
© 2019 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
JH and CK are employees of AOP. HG, VBA, and SB received honoraria and consulted AOP.
Figures
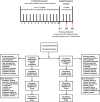
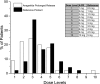
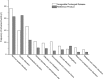
References
-
- Abe Andes, W. , Noveck, R.J. & Fleming, J.S. (1984) Inhibition of platelet production induced by an antiplatelet drug, anagrelide, in normal volunteers. Thrombosis and Haemostasis, 52, 325–328. - PubMed
-
- Ahluwalia, M. , Butcher, L. , Donovan, H. , Killick‐Cole, C. , Jones, P.M. & Erusalimsky, J.D. (2015) The gene expression signature of anagrelide provides an insight into its mechanism of action and uncovers new regulators of megakaryopoiesis. Journal of Thrombosis and Haemostasis, 13, 1103–1112. - PubMed
-
- Barbui, T. (2011) How to manage thrombosis in myeloproliferative neoplasms. Current Opinion in Oncology, 23, 654–658. - PubMed
-
- Barbui, T. , Barosi, G. , Birgegard, G. , Cervantes, F. , Finazzi, G. , Griesshammer, M. , Harrison, C. , Hasselbalch, H.C. , Hehlmann, R. , Hoffman, R. , Kiladjian, J.J. , Kroger, N. , Mesa, R. , McMullin, M.F. , Pardanani, A. , Passamonti, F. , Vannucchi, A.M. , Reiter, A. , Silver, R.T. , Verstovsek, S. , Tefferi, A. & European, L. (2011) Philadelphia‐negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. Journal of Clinical Oncology, 29, 761–770. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

