Long-term caloric restriction ameliorates deleterious effects of aging on white and brown adipose tissue plasticity
- PMID: 30920127
- PMCID: PMC6516146
- DOI: 10.1111/acel.12948
Long-term caloric restriction ameliorates deleterious effects of aging on white and brown adipose tissue plasticity
Abstract
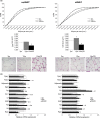
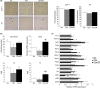
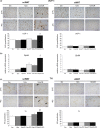
Age-related increased adiposity is an important contributory factor in the development of insulin resistance (IR) and is associated with metabolic defects. Caloric restriction (CR) is known to induce weight loss and to decrease adiposity while preventing metabolic risk factors. Here, we show that moderate 20% CR delays early deleterious effects of aging on white and brown adipose tissue (WAT and BAT, respectively) function and improves peripheral IR. To elucidate the role of CR in delaying early signs of aging, young (3 months), middle-aged (12 months), and old (20 months) mice fed al libitum and middle-aged and old mice subjected to early-onset CR were used. We show that impaired plasticity of subcutaneous WAT (scWAT) contributes to IR, which is already evident in middle-aged mice. Moreover, alteration of thyroid axis status with age is an important factor contributing to BAT dysfunction in middle-aged animals. Both defects in WAT and BAT/beige cells are ameliorated by CR. Accordingly, CR attenuated the age-related decline in scWAT function and decreased the extent of fibro-inflammation. Furthermore, CR promoted scWAT browning. In brief, our study identifies the contribution of scWAT impairment to age-associated metabolic dysfunction and identifies browning in response to food restriction, as a potential therapeutic strategy to prevent the adverse metabolic effects in middle-aged animals.
Keywords: adipose tissue; aging; caloric restriction; fibro-inflammation; insulin resistance; thyroid hormone.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures



References
-
- Arita, Y. , Kihara, S. , Ouchi, N. , Takahashi, M. , Maeda, K. , Miyagawa, J. , … Matsuzawa, Y. (1999). Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications, 257, 79–83. - PubMed
-
- Auffret, J. , Viengchareun, S. , Carre, N. , Denis, R. G. P. , Magnan, C. , Marie, P.‐Y. , … Binart, N. (2012). Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high‐fat‐diet‐induced obesity. The FASEB Journal, 26, 3728–3737. 10.1096/fj.12-204958 - DOI - PubMed
-
- Barger, J. L. , Vann, J. M. , Cray, N. L. , Pugh, T. D. , Mastaloudis, A. , Hester, S. N. , … Prolla, T. A. (2017). Identification of tissue‐specific transcriptional markers of caloric restriction in the mouse and their use to evaluate caloric restriction mimetics. Aging Cell, 16, 750–760. 10.1111/acel.12608 - DOI - PMC - PubMed
-
- Basu, R. , Breda, E. , Oberg, A. L. , Powell, C. C. , Dalla Man, C. , Basu, A. , … Rizza, R. A. (2003). Mechanisms of the age‐associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes, 52, 1738–1748. 10.2337/diabetes.52.7.1738 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- S2010/BMD-2433/Community of Madrid/International
- B2017BMD-3684/Community of Madrid/International
- 2015-CP079/Xunta de Galicia/International
- FPU12/01827/Ministerio de Educación, Cultura y Deporte/International
- BFU2013-47384-R/Spanish Ministry of Economy and Competitiveness/International
- BFU2016-78951-R/Spanish Ministry of Economy and Competitiveness/International
- SAF2012-32491/Spanish Ministry of Economy and Competitiveness/International
- SAF2015-71026-R/Spanish Ministry of Economy and Competitiveness/International
- BFU2015-70454-REDT/Spanish Ministry of Economy and Competitiveness/International
- BFU2017-90578-REDT/Spanish Ministry of Economy and Competitiveness/International
- 281854/European Community's Seventh Framework Programme (FP7/2007-2013)/International

