Thermoresponsive Gels
- PMID: 30920501
- PMCID: PMC6318636
- DOI: 10.3390/gels3010004
Thermoresponsive Gels
Abstract
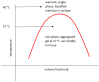
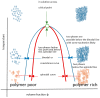
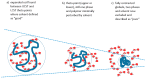
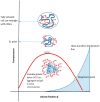
Thermoresponsive gelling materials constructed from natural and synthetic polymers can be used to provide triggered action and therefore customised products such as drug delivery and regenerative medicine types as well as for other industries. Some materials give Arrhenius-type viscosity changes based on coil to globule transitions. Others produce more counterintuitive responses to temperature change because of agglomeration induced by enthalpic or entropic drivers. Extensive covalent crosslinking superimposes complexity of response and the upper and lower critical solution temperatures can translate to critical volume temperatures for these swellable but insoluble gels. Their structure and volume response confer advantages for actuation though they lack robustness. Dynamic covalent bonding has created an intermediate category where shape moulding and self-healing variants are useful for several platforms. Developing synthesis methodology-for example, Reversible Addition Fragmentation chain Transfer (RAFT) and Atomic Transfer Radical Polymerisation (ATRP)-provides an almost infinite range of materials that can be used for many of these gelling systems. For those that self-assemble into micelle systems that can gel, the upper and lower critical solution temperatures (UCST and LCST) are analogous to those for simpler dispersible polymers. However, the tuned hydrophobic-hydrophilic balance plus the introduction of additional pH-sensitivity and, for instance, thermochromic response, open the potential for coupled mechanisms to create complex drug targeting effects at the cellular level.
Keywords: LCST; UCST; drug delivery; hydrogel; micelle; multi-stimulus; organogel; thermoresponsive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



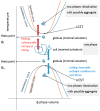
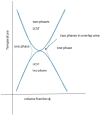

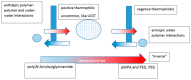
References
-
- Aguilar M.R., San Román J. 1—Introduction to smart polymers and their applications. In: Aguilar M.R., Román J.S., editors. Smart Polymers and Their Applications. Woodhead Publishing; Cambridge, UK: 2014. pp. 1–11.
-
- Zhang J., Zeng L., Feng J. Dynamic covalent gels assembled from small molecules: From discrete gelators to dynamic covalent polymers. Chin. Chem. Lett. 2016 doi: 10.1016/j.cclet.2016.07.015. in press. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

