Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin
- PMID: 30920505
- PMCID: PMC6318674
- DOI: 10.3390/gels3010008
Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin
Abstract
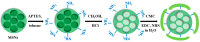
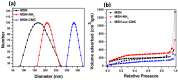
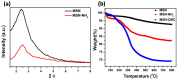
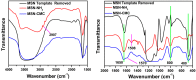
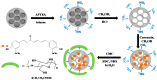
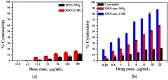
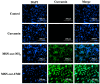
Mesoporous silica nanoparticles (MSNs) with ordered pore structure have been synthesized and used as carriers for the anticancer drug curcumin. MSNs were functionalized with amine groups and further attached with carboxymethyl cellulose (CMC) using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) coupling chemistry, which increased the hydrophilicity and biocompatibility of MSNs. The functionalized MSNs (MSN-NH₂ and MSN-CMC) were characterized using Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Dynamic Light Scattering (DLS), N₂ adsorption, X-Ray Diffraction (XRD), Thermo Gravimetric Analysis (TGA) and Fourier Transform Infrared Spectroscopy (FT-IR). The in vitro release of curcumin from the ⁻NH₂ and CMC functionalized MSNs (MSN-cur-NH₂ and MSN-cur-CMC) was performed in 0.5% aqueous solution of sodium lauryl sulphate (SLS). The effect of CMC functionalization of MSNs towards cellular uptake was studied in the human breast cancer cell line MDA-MB-231 and was compared with that of MSN-NH₂ and free curcumin (cur). Both MSN-NH₂ and MSN-CMC showed good biocompatibility with the breast cancer cell line. The MTT assay study revealed that curcumin-loaded MSN-cur-CMC showed better uptake as compared to curcumin-loaded MSN-cur-NH₂. Free curcumin was used as a control and was shown to have much less internalization as compared to the curcumin-loaded functionalized MSNs due to poor bioavailability. Fluorescence microscopy was used to localize the fluorescent drug curcumin inside the cells. The work demonstrates that CMC-functionalized MSNs can be used as potential carriers for loading and release of hydrophobic drugs that otherwise cannot be used effectively in their free form for cancer therapy.
Keywords: carboxymethyl cellulose; curcumin; drug delivery; mesoporous silica nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

