Properties of Water Bound in Hydrogels
- PMID: 30920534
- PMCID: PMC6318700
- DOI: 10.3390/gels3040037
Properties of Water Bound in Hydrogels
Abstract
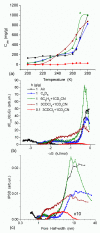
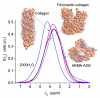
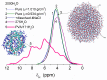
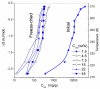
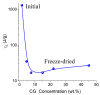
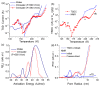
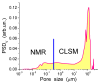
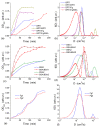
In this review, the importance of water in hydrogel (HG) properties and structure is analyzed. A variety of methods such as ¹H NMR (nuclear magnetic resonance), DSC (differential scanning calorimetry), XRD (X-ray powder diffraction), dielectric relaxation spectroscopy, thermally stimulated depolarization current, quasi-elastic neutron scattering, rheometry, diffusion, adsorption, infrared spectroscopy are used to study water in HG. The state of HG water is rather non-uniform. According to thermodynamic features of water in HG, some of it is non-freezing and strongly bound, another fraction is freezing and weakly bound, and the third fraction is non-bound, free water freezing at 0 °C. According to structural features of water in HG, it can be divided into two fractions with strongly associated and weakly associated waters. The properties of the water in HG depend also on the amounts and types of solutes, pH, salinity, structural features of HG functionalities.
Keywords: cryogels; freezing-melting point depression; hydrogels; interfacial phenomena; strongly and weakly associated water; strongly and weakly bound water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
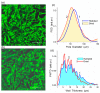
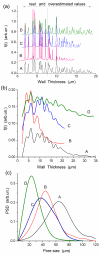

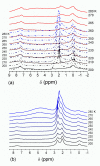
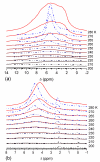


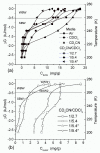
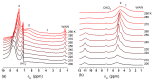

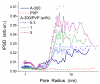
References
-
- Jayakumar R., Menon D., Manzoor K., Nair S.V., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010;82:227–232. doi: 10.1016/j.carbpol.2010.04.074. - DOI
-
- Zhang Y.-F., Yin P., Zhao X.-Q., Wang J., Wang C.D., Ren L., Zhang Q.Q. O-Carboxymethyl-chitosan/organosilica hybrid nanoparticles as non-viral vectors for gene delivery. Mater. Sci. Eng. C. 2009;29:2045–2049. doi: 10.1016/j.msec.2009.04.003. - DOI
-
- Long Y.-Z., Li M.-M., Gu C., Wan M., Duvail J.-L., Liu Z., Fan Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011;36:1415–1442. doi: 10.1016/j.progpolymsci.2011.04.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

