Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial
- PMID: 30920593
- PMCID: PMC6567829
- DOI: 10.1001/jamaoncol.2019.0096
Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial
Abstract
Importance: In retrospective studies, 68Ga-PSMA-11 positron emission tomographic (PET) imaging improves detection of biochemically recurrent prostate cancer compared with conventional imaging.
Objective: To assess 68Ga-PSMA-11 PET accuracy in a prospective multicenter trial.
Design, setting, and participants: In this single-arm prospective trial conducted at University of California, San Francisco and University of California, Los Angeles, 635 patients with biochemically recurrent prostate cancer after prostatectomy (n = 262, 41%), radiation therapy (n = 169, 27%), or both (n = 204, 32%) underwent 68Ga-PSMA-11 PET. Presence of prostate cancer was recorded by 3 blinded readers on a per-patient and per-region base. Lesions were validated by histopathologic analysis and a composite reference standard.
Main outcomes and measures: Endpoints were positive predictive value (PPV), detection rate, interreader reproducibility, and safety.
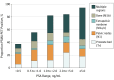
Results: A total of 635 men were enrolled with a median age of 69 years (range, 44-95 years). On a per-patient basis, PPV was 0.84 (95% CI, 0.75-0.90) by histopathologic validation (primary endpoint, n = 87) and 0.92 (95% CI, 0.88-0.95) by the composite reference standard (n = 217). 68Ga-PSMA-11 PET localized recurrent prostate cancer in 475 of 635 (75%) patients; detection rates significantly increased with prostate-specific antigen (PSA): 38% for <0.5 ng/mL (n = 136), 57% for 0.5 to <1.0 ng/mL (n = 79), 84% for 1.0 to <2.0 ng/mL (n = 89), 86% for 2.0 to <5.0 ng/mL (n = 158), and 97% for ≥5.0 ng/mL (n = 173, P < .001). Interreader reproducibility was substantial (Fleiss κ, 0.65-0.78). There were no serious adverse events associated with 68Ga-PSMA-11 administration. PET-directed focal therapy alone led to a PSA drop of 50% or more in 31 of 39 (80%) patients.
Conclusions and relevance: Using blinded reads and independent lesion validation, we establish high PPV for 68Ga-PSMA-11 PET, detection rate and interreader agreement for localization of recurrent prostate cancer.
Trial registration: ClinicalTrials.gov identifiers: NCT02940262 and NCT03353740.
Conflict of interest statement
Figures
Comment in
-
68Ga-PSMA-11 PET enables accurate detection of recurrent disease.Nat Rev Clin Oncol. 2019 Jul;16(7):403. doi: 10.1038/s41571-019-0210-8. Nat Rev Clin Oncol. 2019. PMID: 30952962 No abstract available.
-
68Ga-PSMA-11 PET enables accurate detection of recurrent disease.Nat Rev Urol. 2019 Jul;16(7):388. doi: 10.1038/s41585-019-0181-7. Nat Rev Urol. 2019. PMID: 30971764 No abstract available.
-
Re: Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial.Eur Urol. 2019 Oct;76(4):538-539. doi: 10.1016/j.eururo.2019.05.016. Epub 2019 May 23. Eur Urol. 2019. PMID: 31130433 No abstract available.
-
Editorial: assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer, a prospective single arm clinical study: California shows the way!Transl Androl Urol. 2019 Jul;8(Suppl 3):S296-S299. doi: 10.21037/tau.2019.06.11. Transl Androl Urol. 2019. PMID: 31392151 Free PMC article. No abstract available.
-
68Ga-PSMA-11 PET accuracy in recurrent prostate cancer.Transl Androl Urol. 2019 Dec;8(6):772-774. doi: 10.21037/tau.2019.07.13. Transl Androl Urol. 2019. PMID: 32038977 Free PMC article. No abstract available.
References
-
- Association AU. PSA Testing for the Pretreatment Staging and Posttreatment Management of Prostate Cancer. http://www.auanet.org/documents//education/clinical-guidance/Prostate-Sp.... Accessed July 27, 2018.
-
- Network NCC. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Prostate Cancer (Version 4.2018). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed July 27, 2018.
-
- Mottet N, van den Bergh RCN, Briers E, et al. EAU Guidelines. Edn. presented at the EAU Annual Congress Copenhagen 2018. ISBN 978-94-92671-01-1. 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

