Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study
- PMID: 30920645
- PMCID: PMC6618133
- DOI: 10.1002/cncr.32116
Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study
Abstract
Background: Inotuzumab ozogamicin (InO) is an antibody-drug conjugate used for adults with relapsed/refractory B-cell precursor (BCP) acute lymphoblastic leukemia (ALL). The INotuzumab Ozogamicin trial to inVestigAte Tolerability and Efficacy (INO-VATE) previously reported improved outcomes with InO versus standard-of-care (SoC) chemotherapy. This article reports the final INO-VATE results (≥2 years of follow-up) and additional analyses of patient characteristics associated with improved outcomes.
Methods: Between August 27, 2012, and January 4, 2015, this multicenter, parallel, open-label, phase 3 trial randomized 326 adults with relapsed/refractory ALL to InO (n = 164) or SoC (n = 162); 307 received 1 or more doses of the study drug (164 in the InO arm and 143 in the SoC arm).
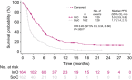
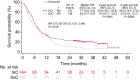
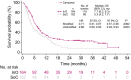
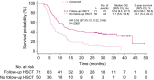
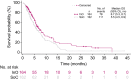
Results: The complete remission (CR)/complete remission with incomplete hematologic recovery (CRi) rate was higher with InO versus SoC (73.8% vs 30.9%; 1-sided P < .0001), with consistent CR/CRi rates across patient subgroups. The median overall survival (OS) was 7.7 months with InO and 6.2 months with SoC, with 2-year OS rates of 22.8% and 10.0%, respectively (overall hazard ratio, 0.75; 97.5% confidence interval [CI], 0.57-0.99; 1-sided P = .0105). The predictors of OS with InO were the best minimal residual disease status, baseline platelet count, duration of first remission, achievement of CR/CRi, and follow-up hematopoietic stem cell transplantation (HSCT; all 2-sided P values < .05). More InO arm patients proceeded directly to HSCT after achieving CR/CRi before any follow-up induction therapy (39.6% [95% CI, 32.1%-47.6%] vs 10.5% [6.2%-16.3%]; 1-sided P < .0001). The most frequent all-grade and grade 3 or higher adverse events in both arms were hematologic. Veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) was more frequent with InO (23 of 164 [14.0%] vs 3 of 143 [2.1%]).
Conclusions: In patients with relapsed/refractory BCP ALL in INO-VATE, InO was associated with a greater likelihood of CR/CRi across key patient subgroups, and it served as a bridge to HSCT. Potential VOD/SOS risk factors must be considered when InO treatment decisions are being made.
Trial registration: ClinicalTrials.gov NCT01564784.
Keywords: acute lymphoblastic leukemia; adults; hematopoietic stem cell transplantation; hepatic veno-occlusive disease; inotuzumab ozogamicin; remission induction.
© 2019 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Conflict of interest statement
Hagop M. Kantarjian declares honoraria from AbbVie, Actinium, Agios, Amgen, ImmunoGen, Orsinex, Pfizer, and Takeda and research support from AbbVie, Agios, Amgen, ARIAD, Astex, Bristol‐Myers Squibb, Cyclacel, Daiichi‐Sankyo, ImmunoGen, Jazz Pharmaceuticals, Novartis, and Pfizer. Daniel J. DeAngelo declares honoraria from Amgen, ARIAD, Bristol‐Myers Squibb, Incyte, Novartis, and Pfizer and research support from ARIAD. Matthias Stelljes declares research support and honoraria from Pfizer. Michaela Liedtke declares honoraria from Pfizer. Wendy Stock declares honoraria from Agios, Astellas, Jazz Pharmaceuticals, and Kite. Nicola Gökbuget declares research support and honoraria from Amgen, Novartis, and Pfizer and honoraria from Celgene. Susan M. O’Brien declares honoraria from Pfizer. Elias Jabbour declares research support and consulting fees from AbbVie, Amgen, Pfizer, and Takeda. Tao Wang, Jane Liang White, Barbara Sleight, and Erik Vandendries are employees of and own stock in Pfizer. Anjali S. Advani declares consulting fees and honoraria from Pfizer.
Figures


References
-
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA‐94 trial. Leukemia. 2007;21:1907‐1914. - PubMed
-
- Gokbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032‐2041. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380:45‐56. - PubMed

