Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review
- PMID: 30920776
- PMCID: PMC6438416
- DOI: 10.1002/jcsm.12402
Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review
Abstract
Background: Cachexia has significant impact on the patients' quality of life and prognosis. It is frequently observed in patients with cancer, especially in advanced stages, but prevalence data for the overall population are lacking. Good quality estimates of cancer cachexia in general and for each of the major cancer types would be highly relevant for potential treatment development efforts in this field. Both the USA and European Union (EU) have implemented special clinical development rules for such rare disorders what are called 'orphan diseases'. The cut-off level for a disease to be considered an orphan disease in the USA is 200 000 people (0.06% of the population) and EU is 5 per 10 000 people (0.05% of the population).
Methods: For this systematic review, we searched at PubMed (from inception to 31 January 2018) to identify clinical studies that assessed the prevalence of cachexia in cancer patients at risk. Studies reporting the prevalence of either cancer cachexia or wasting disease in the top-10 cancer types and 4 other selected cancer types known to be particularly commonly complicated by cachexia were included in this analysis (i.e. prostate cancer, breast cancer, colorectal cancer, melanoma, endometrial cancer, thyroid cancer, urinary bladder cancer, non-hodgkin lymphoma, lung cancer, kidney and renal pelvis cancer, head and neck cancer, gastric cancer, liver cancer, and pancreatic cancer). We calculated the current burden of cancer cachexia, disease by disease, in the USA and in the EU and compared them to the current guidelines for the definition of orphan disease status.
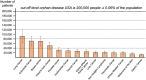
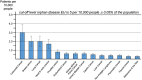
Results: We estimate that in 2014 in the USA, a total of 527 100 patients (16.5 subjects per 10 000 people of the total population), and in 2013 in the EU, a total of 800 300 patients (15.8 subjects per 10 000 people of the total population) suffered from cancer cachexia (of any kind). In the 14 separately analysed cancer types, the prevalence of cancer cachexia in the USA ranged between 11 300 (0.4/10 000, gastric cancer) and 92 000 patients (2.9/10 000, lung cancer) and in the EU between 14 300 (0.3/10 000, melanoma of the skin) and 150 100 (3.0/10 000, colorectal cancer).
Conclusions: The absolute number of patients affected by cancer cachexia in each cancer group is lower than the defined thresholds for orphan diseases in the USA and EU. Cancer cachexia in each subgroup separately should be considered an orphan disease.
Keywords: Cachexia; Epidemiology; European Union; Orphan disease; Prevalence; USA.
© 2019 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
M.M., W.H., A.J., F.S., and U.L. report no conflicts of interest. M.S.A. reports receiving personal fees from Servier. R.H. reports consultancy for bioAffinity, CranioVation, Erbe Medical, and iCAD. S.v.H. reports consultancy for Novartis, Helsinn, Bayer, Respicardia, Vifor Pharma, and Chugai. J.E.M. reports consultancy for Boehringer Ingelheim and Abbott nutrition. A.J.S.C. reports consultancy for Respicardia, Vifor, and Actimed Therapeutics. S.D.A. reports consultancy and/or speaking for Vifor International, Novartis, Servier, Helsinn, Bayer, Boehringer Ingelheim, and Actimed Therapeutics. S.D.A. reports grant support for clinical trials from Vifor International and Abbott. A.J.S.C. and S.D.A. report owning shares of Actimed Therapeutics.
Figures

Comment in
-
Cancer cachexia: an orphan with a future.J Cachexia Sarcopenia Muscle. 2019 Feb;10(1):3-5. doi: 10.1002/jcsm.12401. J Cachexia Sarcopenia Muscle. 2019. PMID: 30920780 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

