Intestinal serine protease inhibition increases FGF21 and improves metabolism in obese mice
- PMID: 30920846
- PMCID: PMC7054636
- DOI: 10.1152/ajpgi.00404.2018
Intestinal serine protease inhibition increases FGF21 and improves metabolism in obese mice
Abstract
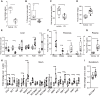
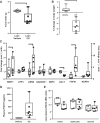
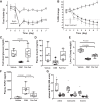
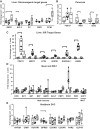
Trypsin is the major serine protease responsible for intestinal protein digestion. An inhibitor, camostat (CS), reduced weight gain, hyperglycemia, and dyslipidemia in obese rats; however, the mechanisms for these are largely unknown. We reasoned that CS creates an apparent dietary protein restriction, which is known to increase hepatic fibroblast growth factor 21 (FGF21). Therefore, metabolic responses to CS and a gut-restricted CS metabolite, FOY-251, were measured in mice. Food intake, body weight, blood glucose, branched-chain amino acids (LC/MS), hormone levels (ELISA), liver pathology (histology), and transcriptional changes (qRT-PCR) were measured in ob/ob, lean and diet-induced obese (DIO) C57BL/6 mice. In ob/ob mice, CS in chow (9-69 mg/kg) or FOY-251 (46 mg/kg) reduced food intake and body weight gain to a similar extent as pair-fed mice. CS decreased blood glucose, liver weight, and lipidosis and increased FGF21 gene transcription and plasma levels. In lean mice, CS increased liver FGF21 mRNA and plasma levels. Relative to pair feeding, FOY-251 also increased plasma FGF21 and induced liver FGF21 and integrated stress response (ISR) transcription. In DIO mice, FOY-251 (100 mg/kg po) did not alter peak glucose levels but reduced the AUC of the glucose excursion in response to an oral glucose challenge. FOY-251 increased plasma FGF21 levels. In addition to previously reported satiety-dependent (cholecystokinin-mediated) actions, intestinal trypsin inhibition engages non-satiety-related pathways in both leptin-deficient and DIO mice. This novel mechanism improves metabolism by a liver-integrated stress response and increased FGF21 expression levels in mice. NEW & NOTEWORTHY Trypsin inhibitors, including plant-based consumer products, have long been associated with metabolic improvements. Studies in the 1980s and 1990s suggested this was due to satiety hormones and caloric wasting by loss of protein and fatty acids in feces. This work suggests an entirely new mechanism based on the lower amounts of digested protein available in the gut. This apparent protein reduction may cause beneficial metabolic adaptation by the intestinal-liver axis to perceived nutrient stress.
Keywords: camostat; gluconeogenesis; integrated stress response; liver; obesity; protein dilution; small intestine; triglycerides; type 2 diabetes.
Conflict of interest statement
All coauthors were employed by Janssen Pharmaceutical R&D during these studies.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

