Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial
- PMID: 30920880
- PMCID: PMC6638596
- DOI: 10.1200/JCO.18.02122
Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial
Abstract
Purpose: This trial aimed to assess the efficacy and safety of the paclitaxel plus fluorouracil regimen versus the cisplatin plus fluorouracil regimen in definitive concurrent chemoradiotherapy (dCRT) in patients with locally advanced esophageal squamous cell carcinoma (ESCC).
Patients and methods: Patients with locally advanced ESCC were enrolled and randomly assigned to either the paclitaxel plus fluorouracil group or the cisplatin plus fluorouracil group. The patients in the paclitaxel plus fluorouracil group were treated with paclitaxel and fluorouracil one cycle per week in dCRT for five cycles followed by paclitaxel and fluorouracil one cycle per month in consolidation chemotherapy for two cycles. The patients in the cisplatin/5-fluorouracil group were treated with cisplatin and fluorouracil one cycle per month in dCRT for two cycles followed by two cycles in consolidation chemotherapy. The radiotherapy dose was 61.2 Gy delivered in 34 fractions. The primary end point was 3-year overall survival (OS).
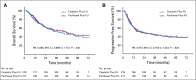
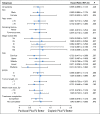
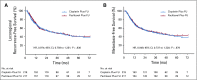
Results: Four hundred thirty-six patients with ESCC in six centers were recruited at a 1:1 ratio between April 2012 and July 2015. The median follow-up of the surviving patients was 48.7 months (interquartile range, 42.6-60.9). The 3-year OS was 55.4% in the paclitaxel plus fluorouracil group and 51.8% in the cisplatin plus fluorouracil group (hazard ratio, 0.905 [95% CI, 0.698 to 1.172]; P = .448). The 3-year progression-free survival was also not significantly different between the paclitaxel plus fluorouracil group and the cisplatin plus fluorouracil group (43.7% v 45.5%, respectively; hazard ratio, 0.973 [95% CI, 0.762 to 1.243]; P = .828). Compared with the cisplatin plus fluorouracil group, the paclitaxel plus fluorouracil group had significantly lower incidences of acute grade 3 or higher anemia, thrombocytopenia, anorexia, nausea, vomiting, and fatigue (P < .05), but higher incidences of acute grade 3 or higher leukopenia, radiation dermatitis, and radiation pneumonitis (P < .05).
Conclusion: The paclitaxel plus fluorouracil regimen did not significantly prolong the OS compared with the standard cisplatin plus fluorouracil regimen in dCRT in patients with locally advanced ESCC.
Trial registration: ClinicalTrials.gov NCT01591135.
Figures

Comment in
-
Reply to A. Adenis et al.J Clin Oncol. 2019 Sep 10;37(26):2380-2381. doi: 10.1200/JCO.19.01423. Epub 2019 Jul 29. J Clin Oncol. 2019. PMID: 31356141 No abstract available.
-
Definitive Chemoradiotherapy for Esophageal Squamous Cell Cancer: A Matter of Standard.J Clin Oncol. 2019 Sep 10;37(26):2379. doi: 10.1200/JCO.19.00963. Epub 2019 Jul 29. J Clin Oncol. 2019. PMID: 31356142 No abstract available.
-
Taxane- Versus Cisplatin-Based Chemotherapy With Radiation Therapy Is a Better Platform to Refine Esophageal Cancer Therapy.J Clin Oncol. 2019 Oct 20;37(30):2805-2806. doi: 10.1200/JCO.19.01247. Epub 2019 Aug 29. J Clin Oncol. 2019. PMID: 31465257 No abstract available.
References
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01) JAMA. 1999;281:1623–1627. - PubMed
-
- Tishler RB, Schiff PB, Geard CR, et al. Taxol: A novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22:613–617. - PubMed
-
- Ajani JA, Ilson DH, Daugherty K, et al. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst. 1994;86:1086–1091. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

