Limited differentiation among Plasmodium vivax populations from the northwest and to the south Pacific Coast of Colombia: A malaria corridor?
- PMID: 30921317
- PMCID: PMC6456216
- DOI: 10.1371/journal.pntd.0007310
Limited differentiation among Plasmodium vivax populations from the northwest and to the south Pacific Coast of Colombia: A malaria corridor?
Abstract
Background: Malaria remains endemic in several countries of South America with low to moderate transmission intensity. Regional human migration through underserved endemic areas may be responsible for significant parasite dispersion making the disease resilient to interventions. Thus, the genetic characterization of malarial parasites is an important tool to assess how endemic areas may connect via the movement of infected individuals. Here, four sites in geographically separated areas reporting 80% of the malaria morbidity in Colombia were studied. The sites are located on an imaginary transect line of 1,500 km from the northwest to the south Pacific Coast of Colombia with a minimal distance of 500 km between populations that display noticeable ethnic, economic, epidemiological, and ecological differences.
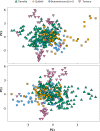
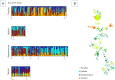
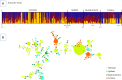
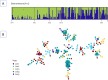
Methodology/principal findings: A total of 624 Plasmodium vivax samples from the four populations were genotyped by using eight microsatellite loci. Although a strong geographic structure was expected between these populations, only moderate evidence of genetic differentiation was observed using a suite of population genetic analyses. High genetic diversity, shared alleles, and low linkage disequilibrium were also found in these P. vivax populations providing no evidence for a bottleneck or clonal expansions as expected from recent reductions in the transmission that could have been the result of scaling up interventions or environmental changes. These patterns are consistent with a disease that is not only endemic in each site but also imply that there is gene flow among these populations across 1,500 km.
Conclusion /significance: The observed patterns in P. vivax are consistent with a "corridor" where connected endemic areas can sustain a high level of genetic diversity locally and can restore parasite-subdivided populations via migration of infected individuals even after local interventions achieved a substantial reduction of clinical cases. The consequences of these findings in terms of control and elimination are discussed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Nationwide genetic surveillance of Plasmodium vivax in Papua New Guinea reveals heterogeneous transmission dynamics and routes of migration amongst subdivided populations.Infect Genet Evol. 2018 Mar;58:83-95. doi: 10.1016/j.meegid.2017.11.028. Epub 2017 Dec 5. Infect Genet Evol. 2018. PMID: 29313805
-
Plasmodium vivax Diversity and Population Structure across Four Continents.PLoS Negl Trop Dis. 2015 Jun 30;9(6):e0003872. doi: 10.1371/journal.pntd.0003872. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26125189 Free PMC article.
-
Dynamics of Plasmodium vivax populations in border areas of the Greater Mekong sub-region during malaria elimination.Malar J. 2020 Apr 8;19(1):145. doi: 10.1186/s12936-020-03221-9. Malar J. 2020. PMID: 32268906 Free PMC article.
-
Uncovering the transmission dynamics of Plasmodium vivax using population genetics.Pathog Glob Health. 2015 May;109(3):142-52. doi: 10.1179/2047773215Y.0000000012. Epub 2015 Apr 18. Pathog Glob Health. 2015. PMID: 25891915 Free PMC article. Review.
-
Understanding the population genetics of Plasmodium vivax is essential for malaria control and elimination.Malar J. 2012 Jan 10;11:14. doi: 10.1186/1475-2875-11-14. Malar J. 2012. PMID: 22233585 Free PMC article. Review.
Cited by
-
Microsatellite analysis reveals connectivity among geographically distant transmission zones of Plasmodium vivax in the Peruvian Amazon: A critical barrier to regional malaria elimination.PLoS Negl Trop Dis. 2019 Nov 11;13(11):e0007876. doi: 10.1371/journal.pntd.0007876. eCollection 2019 Nov. PLoS Negl Trop Dis. 2019. PMID: 31710604 Free PMC article.
-
Bias-corrected maximum-likelihood estimation of multiplicity of infection and lineage frequencies.PLoS One. 2021 Dec 29;16(12):e0261889. doi: 10.1371/journal.pone.0261889. eCollection 2021. PLoS One. 2021. PMID: 34965279 Free PMC article.
-
High-resolution micro-epidemiology of parasite spatial and temporal dynamics in a high malaria transmission setting in Kenya.Nat Commun. 2019 Dec 9;10(1):5615. doi: 10.1038/s41467-019-13578-4. Nat Commun. 2019. PMID: 31819062 Free PMC article.
-
PyPop: a mature open-source software pipeline for population genomics.Front Immunol. 2024 Apr 2;15:1378512. doi: 10.3389/fimmu.2024.1378512. eCollection 2024. Front Immunol. 2024. PMID: 38629078 Free PMC article.
-
Balancing selection and high genetic diversity of Plasmodium vivax circumsporozoite central region in parasites from Brazilian Amazon and Rio de Janeiro Atlantic Forest.PLoS One. 2020 Nov 9;15(11):e0241426. doi: 10.1371/journal.pone.0241426. eCollection 2020. PLoS One. 2020. PMID: 33166298 Free PMC article.
References
-
- World Health Organization (WHO). A framework for malaria elimination 2017;100. http://www.who.int/malaria/publications/atoz/9789241511988/en/
-
- World Health Organization (WHO). World malaria report 2017;160. http://www.who.int/malaria/publications/world-malaria-report-2017/en/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

