PKC regulates the production of fibroblast growth factor 23 (FGF23)
- PMID: 30921339
- PMCID: PMC6438472
- DOI: 10.1371/journal.pone.0211309
PKC regulates the production of fibroblast growth factor 23 (FGF23)
Abstract
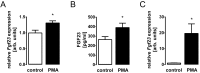
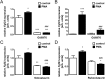
Serine/threonine protein kinase C (PKC) is activated by diacylglycerol that is released from membrane lipids by phospholipase C in response to activation of G protein-coupled receptors or receptor tyrosine kinases. PKC isoforms are particularly relevant for proliferation and differentiation of cells including osteoblasts. Osteoblasts/osteocytes produce fibroblast growth factor 23 (FGF23), a hormone regulating renal phosphate and vitamin D handling. PKC activates NFκB, a transcription factor complex controlling FGF23 expression. Here, we analyzed the impact of PKC on FGF23 synthesis. Fgf23 expression was analyzed by qRT-PCR in UMR106 osteoblast-like cells and in IDG-SW3 osteocytes, and FGF23 protein was measured by ELISA. Phorbol ester 12-O-tetradecanoylphorbol-13-acetate (PMA), a PKC activator, up-regulated FGF23 production. In contrast, PKC inhibitors calphostin C, Gö6976, sotrastaurin and ruboxistaurin suppressed FGF23 formation. NFκB inhibitor withaferin A abolished the stimulatory effect of PMA on Fgf23. PKC is a powerful regulator of FGF23 synthesis, an effect which is at least partly mediated by NFκB.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


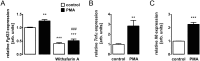
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

