Effect of impregnated central venous catheters on thrombosis in paediatric intensive care: Post-hoc analyses of the CATCH trial
- PMID: 30921401
- PMCID: PMC6438638
- DOI: 10.1371/journal.pone.0214607
Effect of impregnated central venous catheters on thrombosis in paediatric intensive care: Post-hoc analyses of the CATCH trial
Abstract
Purpose: The CATheter infections in CHildren (CATCH) trial reported reduced risks of bloodstream infection with antibiotic impregnated compared with heparin-bonded or standard central venous catheters (CVC) in paediatric intensive care. CVC impregnation did not increase the risk of thrombosis which was recorded in 24% of participants. This post-hoc analysis determines the effect of CVC impregnation on the risk of thrombosis leading to CVC removal or swollen limb.
Methods: We analysed patients in the CATCH trial, blind to CVC allocation, to define clinically relevant thrombosis based on the clinical sign most frequently recorded in patients where the CVC was removed because of concerns regarding thrombosis. In post-hoc, three-way comparisons of antibiotic, heparin and standard CVCs, we determined the effect of CVC type on time to clinically relevant thrombosis, using Cox proportional hazards regression.
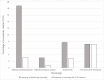
Results: Of 1409 participants with a successful CVC insertion, the sign most frequently resulting in CVC removal was swollen limb (37.6%; 41/109), with lower rates of removal of CVC following 2 episodes of difficulty withdrawing blood or of flushing to unblock the CVC. In intention to treat analyses (n = 1485), clinically relevant thrombosis, defined by 1 or more record of swollen limb or CVC removal due to concerns about thrombosis, was recorded in 11.9% (58/486) of antibiotic CVCs, 12.1% (60/497) of heparin CVCs, and 10.2% (51/502) of standard CVCs. We found no differences in time to clinically relevant thrombosis according to type of CVC.
Conclusions: We found no evidence for an increased risk of clinically relevant thrombosis in antibiotic impregnated compared to heparin-bonded or standard CVCs in children receiving intensive care.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
CATheter Infections in CHildren (CATCH): a randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children.Health Technol Assess. 2016 Mar;20(18):vii-xxviii, 1-219. doi: 10.3310/hta20180. Health Technol Assess. 2016. PMID: 26935961 Free PMC article. Clinical Trial.
-
Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial.Lancet. 2016 Apr 23;387(10029):1732-42. doi: 10.1016/S0140-6736(16)00340-8. Epub 2016 Mar 4. Lancet. 2016. PMID: 26946925 Clinical Trial.
-
Comparison of triple-lumen central venous catheters impregnated with silver nanoparticles (AgTive®) vs conventional catheters in intensive care unit patients.J Hosp Infect. 2012 Oct;82(2):101-7. doi: 10.1016/j.jhin.2012.07.010. Epub 2012 Aug 28. J Hosp Infect. 2012. PMID: 22938728 Clinical Trial.
-
Normal saline (0.9% sodium chloride) versus heparin intermittent flushing for the prevention of occlusion in long-term central venous catheters in infants and children.Cochrane Database Syst Rev. 2020 Apr 30;4(4):CD010996. doi: 10.1002/14651858.CD010996.pub3. Cochrane Database Syst Rev. 2020. PMID: 32352563 Free PMC article.
-
Low molecular weight heparin for prevention of central venous catheter-related thrombosis in children.Cochrane Database Syst Rev. 2020 Jun 18;6(6):CD005982. doi: 10.1002/14651858.CD005982.pub3. Cochrane Database Syst Rev. 2020. PMID: 32557627 Free PMC article.
Cited by
-
Peripherally inserted central catheter design and material for reducing catheter failure and complications.Cochrane Database Syst Rev. 2024 Jun 28;6(6):CD013366. doi: 10.1002/14651858.CD013366.pub2. Cochrane Database Syst Rev. 2024. PMID: 38940297 Free PMC article.
References
-
- Higgerson RA, Lawson KA, Christie LM, Brown AM, McArthur JA, Totapally BR, et al. Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2011;12(6):628–34. 10.1097/PCC.0b013e318207124a - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical