Targeting ferroptosis: A novel therapeutic strategy for the treatment of mitochondrial disease-related epilepsy
- PMID: 30921410
- PMCID: PMC6438538
- DOI: 10.1371/journal.pone.0214250
Targeting ferroptosis: A novel therapeutic strategy for the treatment of mitochondrial disease-related epilepsy
Abstract
Background: Mitochondrial disease is a family of genetic disorders characterized by defects in the generation and regulation of energy. Epilepsy is a common symptom of mitochondrial disease, and in the vast majority of cases, refractory to commonly used antiepileptic drugs. Ferroptosis is a recently-described form of iron- and lipid-dependent regulated cell death associated with glutathione depletion and production of lipid peroxides by lipoxygenase enzymes. Activation of the ferroptosis pathway has been implicated in a growing number of disorders, including epilepsy. Given that ferroptosis is regulated by balancing the activities of glutathione peroxidase-4 (GPX4) and 15-lipoxygenase (15-LO), targeting these enzymes may provide a rational therapeutic strategy to modulate seizure. The clinical-stage therapeutic vatiquinone (EPI-743, α-tocotrienol quinone) was reported to reduce seizure frequency and associated morbidity in children with the mitochondrial disorder pontocerebellar hypoplasia type 6. We sought to elucidate the molecular mechanism of EPI-743 and explore the potential of targeting 15-LO to treat additional mitochondrial disease-associated epilepsies.
Methods: Primary fibroblasts and B-lymphocytes derived from patients with mitochondrial disease-associated epilepsy were cultured under standardized conditions. Ferroptosis was induced by treatment with the irreversible GPX4 inhibitor RSL3 or a combination of pharmacological glutathione depletion and excess iron. EPI-743 was co-administered and endpoints, including cell viability and 15-LO-dependent lipid oxidation, were measured.
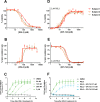
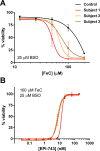
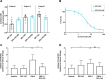
Results: EPI-743 potently prevented ferroptosis in patient cells representing five distinct pediatric disease syndromes with associated epilepsy. Cytoprotection was preceded by a dose-dependent decrease in general lipid oxidation and the specific 15-LO product 15-hydroxyeicosatetraenoic acid (15-HETE).
Conclusions: These findings support the continued clinical evaluation of EPI-743 as a therapeutic agent for PCH6 and other mitochondrial diseases with associated epilepsy.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: The following authors declare financial competing interests as paid current or former employees and/or equity holders of BioElectron Technology Corporation, Inc.: AA, JJB, AH, KGH, CRH, AHKK, VK, MBK, YK, JCL, CIM, SAM, JJM, WDS, SS, JKT, LWang. EPI-743 is a commercial product in development by BioElectron Technology Corporation, Inc., from which employees may benefit. The following authors declare patent applications (pending or actual) belonging to BioElectron Technology Corporation, from which they may benefit: JJB, AH, CRH, AHKK, JCL, SAM, WDS, JKT. The following authors declare grant support from BioElectron Technology Corporation pertaining to the work under consideration forpublication: GME, CD-V, DM. WG declares a cooperative research agreement between NHGRI and BioElectron pertaining to the scope of the work under consideration, as well as to activities outside the submitted work. RPS is a site principal investigator for two studies sponsored by BioElectron Technology Corporation. GME is a site principal investigator for an emergency protocol sponsored by BioElectron Technology Corporation. Outside the scope of the submitted work, EAA declares he is a Co-Founder of Personalis Inc., and DeepCell, Inc. CD-V declares grant support from Actelion and Sanofi Genzyme, as well as personal fees from Actelion, CureVac, Moderna Therapeutics, Logic- Biotherapeutics, Nutricia, Orphan Europe, Medifood, Promethera, Sanofi Genzyme, and SOBI. GME declares grant support and serving as site principal investigator for a clinical trial sponsored by Stealth Therapeutics. DM declares personal fees from SOBI. Thefollowing authors declared no competing interests: EB, RC, KAC, SCJ, MTW, LWolfe. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis.Free Radic Biol Med. 2018 Mar;117:45-57. doi: 10.1016/j.freeradbiomed.2018.01.019. Epub 2018 Jan 31. Free Radic Biol Med. 2018. PMID: 29378335
-
Differential cell death decisions in the testis: evidence for an exclusive window of ferroptosis in round spermatids.Mol Hum Reprod. 2019 May 1;25(5):241-256. doi: 10.1093/molehr/gaz015. Mol Hum Reprod. 2019. PMID: 30865280
-
Inactivation of the glutathione peroxidase GPx4 by the ferroptosis-inducing molecule RSL3 requires the adaptor protein 14-3-3ε.FEBS Lett. 2020 Feb;594(4):611-624. doi: 10.1002/1873-3468.13631. Epub 2019 Oct 20. FEBS Lett. 2020. PMID: 31581313
-
Mitochondrial regulation of ferroptosis.J Cell Biol. 2021 Sep 6;220(9):e202105043. doi: 10.1083/jcb.202105043. Epub 2021 Jul 30. J Cell Biol. 2021. PMID: 34328510 Free PMC article. Review.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
Cited by
-
The Interplay between Mitochondrial Dysfunction and Ferroptosis during Ischemia-Associated Central Nervous System Diseases.Brain Sci. 2023 Sep 25;13(10):1367. doi: 10.3390/brainsci13101367. Brain Sci. 2023. PMID: 37891735 Free PMC article. Review.
-
Ferroptosis and Its Role in Epilepsy.Front Cell Neurosci. 2021 Jul 15;15:696889. doi: 10.3389/fncel.2021.696889. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34335189 Free PMC article. Review.
-
Emerging perspectives on mitochondrial dysfunctioning and inflammation in epileptogenesis.Inflamm Res. 2021 Dec;70(10-12):1027-1042. doi: 10.1007/s00011-021-01511-9. Epub 2021 Oct 15. Inflamm Res. 2021. PMID: 34652489
-
Targeting ferroptosis with the lipoxygenase inhibitor PTC-041 as a therapeutic strategy for the treatment of Parkinson's disease.PLoS One. 2024 Sep 18;19(9):e0309893. doi: 10.1371/journal.pone.0309893. eCollection 2024. PLoS One. 2024. PMID: 39292705 Free PMC article.
-
Melatonin Alleviates Age-Associated Endothelial Injury of Atherosclerosis via Regulating Telomere Function.J Inflamm Res. 2021 Dec 11;14:6799-6812. doi: 10.2147/JIR.S329020. eCollection 2021. J Inflamm Res. 2021. PMID: 34924765 Free PMC article.
References
-
- Khurana DS, Salganicoff L, Melvin JJ, Hobdell EF, Valencia I, Hardison HH, et al. Epilepsy and respiratory chain defects in children with mitochondrial encephalopathies. Neuropediatrics [Internet]. 2008. February;39(1):8–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18504675 10.1055/s-2008-1076737 - DOI - PubMed
-
- Rahman S. Mitochondrial disease and epilepsy. Dev Med Child Neurol [Internet]. 2012. May;54(5):397–406. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22283595 10.1111/j.1469-8749.2011.04214.x - DOI - PubMed
-
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell [Internet]. 2017;171(2):273–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28985560 10.1016/j.cell.2017.09.021 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

