Thalamo-cortical network hyperconnectivity in preclinical progranulin mutation carriers
- PMID: 30921613
- PMCID: PMC6438992
- DOI: 10.1016/j.nicl.2019.101751
Thalamo-cortical network hyperconnectivity in preclinical progranulin mutation carriers
Abstract
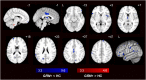
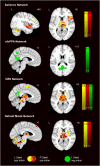
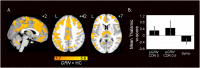
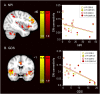
Mutations in progranulin (GRN) cause heterogeneous clinical syndromes, including behavioral variant frontotemporal dementia (bvFTD), primary progressive aphasia (PPA), corticobasal syndrome (CBS) and Alzheimer-type dementia (AD-type dementia). Human studies have shown that presymptomatic GRN carriers feature reduced connectivity in the salience network, a system targeted in bvFTD. Mice with homozygous deletion of GRN, in contrast, show thalamo-cortical hypersynchrony due to aberrant pruning of inhibitory synapses onto thalamo-cortical projection neurons. No studies have systematically explored the intrinsic connectivity networks (ICNs) targeted by the four GRN-associated clinical syndromes, or have forged clear links between human and mouse model findings. We compared 17 preclinical GRN carriers (14 "presymptomatic" clinically normal and three "prodromal" with mild cognitive symptoms) to healthy controls to assess for differences in cognitive testing and gray matter volume. Using task-free fMRI, we assessed connectivity in the salience network, a non-fluent variant primary progressive aphasia network (nfvPPA), the perirolandic network (CBS), and the default mode network (AD-type dementia). GRN carriers and controls showed similar performance on cognitive testing. Although carriers showed little evidence of brain atrophy, markedly enhanced connectivity emerged in all four networks, and thalamo-cortical hyperconnectivity stood out as a unifying feature. Voxelwise assessment of whole brain degree centrality, an unbiased graph theoretical connectivity metric, confirmed thalamic hyperconnectivity. These results show that human GRN disease and the prevailing GRN mouse model share a thalamo-cortical network hypersynchrony phenotype. Longitudinal studies will determine whether this network physiology represents a compensatory response as carriers approach symptom onset, or an early and sustained preclinical manifestation of lifelong progranulin haploinsufficiency.
Keywords: Frontotemporal dementia; GRN; MRI; Progranulin; Thalamus.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Alberici A., Archetti S., Pilotto A., Premi E., Cosseddu M., Bianchetti A., Semeraro F., Salvetti M., Muiesan M.L., Padovani A., Borroni B. Results from a pilot study on amiodarone administration in monogenic frontotemporal dementia with granulin mutation. Neurol. Sci. 2014;35:1215–1219. - PubMed
-
- Armstrong M.J., Litvan I., Lang A.E., Bak T.H., Bhatia K.P., Borroni B., Boxer A.L., Dickson D.W., Grossman M., Hallett M., Josephs K.A., Kertesz A., Lee S.E., Miller B.L., Reich S.G., Riley D.E., Tolosa E., Tröster A.I., Vidailhet M., Weiner W.J. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. - PMC - PubMed
-
- Baker M., Mackenzie I.R., Pickering-Brown S.M., Gass J., Rademakers R., Lindholm C., Snowden J., Adamson J., Sadovnick A.D., Rollinson S., Cannon A., Dwosh E., Neary D., Melquist S., Richardson A., Dickson D., Berger Z., Eriksen J., Robinson T., Zehr C., Dickey C.A., Crook R., McGowan E., Mann D., Boeve B., Feldman H., Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Borroni B., Alberici A., Cercignani M., Premi E., Serra L., Cerini C., Cosseddu M., Pettenati C., Turla M., Archetti S., Gasparotti R., Caltagirone C., Padovani A., Bozzali M. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol. Aging. 2012;33:2506–2520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 AG035610/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R01 AG058233/AG/NIA NIH HHS/United States
- K24 AG045333/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R35 NS097261/NS/NINDS NIH HHS/United States
- U01 AG052943/AG/NIA NIH HHS/United States
- UH3 NS103870/NS/NINDS NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R01 NS100440/NS/NINDS NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- R01 AG026938/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- K23 AG039414/AG/NIA NIH HHS/United States
- R56 NS050915/NS/NINDS NIH HHS/United States
- R01 NS050915/NS/NINDS NIH HHS/United States
- K24 DC015544/DC/NIDCD NIH HHS/United States
- UG3 NS103870/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

