Increased cancer risk in patients undergoing dialysis: a population-based cohort study in North-Eastern Italy
- PMID: 30922296
- PMCID: PMC6437907
- DOI: 10.1186/s12882-019-1283-4
Increased cancer risk in patients undergoing dialysis: a population-based cohort study in North-Eastern Italy
Abstract
Background: In southern Europe, the risk of cancer in patients with end-stage kidney disease receiving dialysis has not been well quantified. The aim of this study was to assess the overall pattern of risk for de novo malignancies (DNMs) among dialysis patients in the Friuli Venezia Giulia region, north-eastern Italy.
Methods: A population-based cohort study among 3407 dialysis patients was conducted through a record linkage between local healthcare databases and the cancer registry (1998-2013). Person-years (PYs) were calculated from 30 days after the date of first dialysis to the date of DNM diagnosis, kidney transplant, death, last follow-up or December 31, 2013, whichever came first. The risk of DNM, as compared to the general population, was estimated using standardized incidence ratios (SIRs) and 95% confidence intervals (CIs).
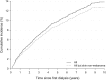
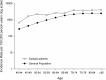
Results: During 10,798 PYs, 357 DNMs were diagnosed in 330 dialysis patients. A higher than expected risk of 1.3-fold was found for all DNMs combined (95% CI: 1.15-1.43). The risk was particularly high in younger dialysis patients (SIR = 1.88, 95% CI: 1.42-2.45 for age 40-59 years), and it decreased with age. Moreover, significantly increased DNM risks emerged during the first 3 years since dialysis initiation, especially within the first year (SIR = 8.52, 95% CI: 6.89-10.41). Elevated excess risks were observed for kidney (SIR = 3.18; 95% CI: 2.06-4.69), skin non-melanoma (SIR = 1.81, 95% CI: 1.46-2.22), oral cavity (SIR = 2.42, 95% CI: 1.36-4.00), and Kaposi's sarcoma (SIR = 10.29, 95% CI: 1.25-37.16).
Conclusions: The elevated risk for DNM herein documented suggest the need to implement a targeted approach to cancer prevention and control in dialysis patients.
Keywords: Cancer risk; Dialysis; End-stage kidney disease; Italy; Population-based study; de novo malignancies.
Conflict of interest statement
Ethics approval and consent to participate
Cancer Registries are identified as collectors of personal data for surveillance purposes without the need of explicit individual consent. The approval of a research ethic committee is not required as this descriptive study was conducted without any direct or indirect intervention on patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures