Modified Dose Efficacy Trial of a Canine Distemper-Measles Vaccine for Use in Rhesus Macaques (Macaca mulatta)
- PMID: 30922419
- PMCID: PMC6526495
- DOI: 10.30802/AALAS-JAALAS-18-000091
Modified Dose Efficacy Trial of a Canine Distemper-Measles Vaccine for Use in Rhesus Macaques (Macaca mulatta)
Abstract
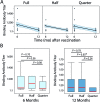
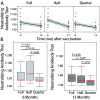
Measles virus causes a highly infectious disease in NHP. Clinical signs range from asymptomatic to fatal, although measles virus is most well-known for its characteristic generalized maculopapular rash. Along with appropriate quarantine practices, restricted human access, and appropriate personal protective equipment, vaccines are used to combat the risk of infection. The canine distemper-measles vaccine (CDMV), administered at the manufacturer's standard dose (1.0 mL IM), has been shown to be effective against clinical measles disease in rhesus macaques (Macaca mulatta). The goal of the current study was to test whether doses smaller than the manufacturer's recommended dose stimulated adequate antibody production to protect against infection. We hypothesized that either 0.25 or 0.5 mL IM of CDMV would stimulate antibody production comparable to the manufacturer's recommended dose. We found that the 0.25-mL dose was less effective at inducing antibodies than either the standard (1.0 mL) or 0.5-mL dose, which both yielded similar titers. The primary implication of this study informs balancing resource allocation and providing efficacious immunity. By using half the manufacturer-recommended dose, the 50% cost reduction may provide sufficient monetary incentive to implement, maintain, or modify measles vaccination programs at NHP facilities.
Figures
Similar articles
-
Comparative efficacy of a canine distemper-measles and a standard measles vaccine for immunization of rhesus macaques (Macaca mulatta).Comp Med. 2002 Oct;52(5):467-72. Comp Med. 2002. PMID: 12405642
-
Canine distemper outbreak in rhesus monkeys, China.Emerg Infect Dis. 2011 Aug;17(8):1541-3. doi: 10.3201/eid1708.101153. Emerg Infect Dis. 2011. PMID: 21801646 Free PMC article.
-
Multicenter Safety and Immunogenicity Trial of an Attenuated Measles Vaccine for NHP.Comp Med. 2015 Oct;65(5):448-54. Comp Med. 2015. PMID: 26473350 Free PMC article.
-
Evaluation of oral and subcutaneous delivery of an experimental canarypox recombinant canine distemper vaccine in the Siberian polecat (Mustela eversmanni).J Zoo Wildl Med. 2003 Mar;34(1):25-35. doi: 10.1638/1042-7260(2003)34[0025:EOOASD]2.0.CO;2. J Zoo Wildl Med. 2003. PMID: 12723797
-
Canine distemper virus.Vet Clin North Am Small Anim Pract. 2008 Jul;38(4):787-97, vii-viii. doi: 10.1016/j.cvsm.2008.02.007. Vet Clin North Am Small Anim Pract. 2008. PMID: 18501278 Review.
Cited by
-
Origin of Canine Distemper Virus: Consolidating Evidence to Understand Potential Zoonoses.Front Microbiol. 2019 Aug 28;10:1982. doi: 10.3389/fmicb.2019.01982. eCollection 2019. Front Microbiol. 2019. PMID: 31555226 Free PMC article. No abstract available.
References
-
- Abee CR, Mansfield K, Tardif SD, Morris T. 2012. Nonhuman primates in biomedical research: diseases. San Francisco (CA): Elsevier Academic Press.
-
- Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
-
- Animal Welfare Regulations. 2013. 9 CFR §3.129.

