For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy
- PMID: 30922619
- PMCID: PMC6524960
- DOI: 10.1016/j.ultrasmedbio.2018.10.035
For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy
Abstract
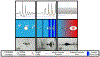
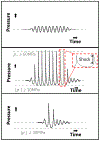
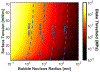
Histotripsy is a focused ultrasound therapy for non-invasive tissue ablation. Unlike thermally ablative forms of therapeutic ultrasound, histotripsy relies on the mechanical action of bubble clouds for tissue destruction. Although acoustic bubble activity is often characterized as chaotic, the short-duration histotripsy pulses produce a unique and consistent type of cavitation for tissue destruction. In this review, the action of histotripsy-induced bubbles is discussed. Sources of bubble nuclei are reviewed, and bubble activity over the course of single and multiple pulses is outlined. Recent innovations in terms of novel acoustic excitations, exogenous nuclei for targeted ablation and histotripsy-enhanced drug delivery and image guidance metrics are discussed. Finally, gaps in knowledge of the histotripsy process are highlighted, along with suggested means to expedite widespread clinical utilization of histotripsy.
Keywords: Ablation; Bubbles; Cavitation; Focused ultrasound; Histotripsy.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures of conflicts of interest
E. Vlaisavljevich is a consultant for HistoSonics (Ann Arbor, MI). A. Maxwell is a consultant and has financial interests with SonoMotion, Inc (South San Francisco, CA).
Figures




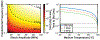



References
-
- Acconcia C, Leung BYC, Hynynen K, Goertz DE. Interactions between ultrasound stimulated microbubbles and fibrin clots. Appl Phys Lett 2013;103:053701.
-
- Ainslie MA, Leighton TG. Review of scattering and extinction cross-sections, damping factors, and resonance frequencies of a spherical gas bubble. J Acoust Soc Am 2011;130:3184. - PubMed
-
- Allen SP, Hall TL, Cain CA, Hernandez-Garcia L. Controlling cavitation-based image contrast in focused ultrasound histotripsy surgery. Magn Reson Med 2014;73:204–213. - PubMed
-
- Allen SP, Hernandez-Garcia L, Cain CA, Hall TL. MR-based detection of individual histotripsy bubble clouds formed in tissues and phantoms. Magn Reson Med 2015;76:1486–1493. - PubMed
-
- Allen SP, Vlaisavljevich E, Shi J, Hernandez-Garcia L, Cain CA, Xu Z, Hall TL. The response of MRI contrast parameters in in vitro tissues and tissue mimicking phantoms to fractionation by histotripsy. Phys Med Biol 2017;62:7167–7180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

