Proactive text messaging (GetReady2Quit) and nicotine replacement therapy to promote smoking cessation among smokers in primary care: A pilot randomized trial protocol
- PMID: 30923022
- PMCID: PMC6571033
- DOI: 10.1016/j.cct.2019.03.006
Proactive text messaging (GetReady2Quit) and nicotine replacement therapy to promote smoking cessation among smokers in primary care: A pilot randomized trial protocol
Abstract
Introduction: Most smokers see a physician each year, but few use any assistance when they try to quit. Text messaging programs improve smoking cessation in community and school settings; however, their efficacy in a primary care setting is unclear. The current trial assesses the feasibility and preliminary clinical outcomes of text messaging and mailed nicotine replacement therapy (NRT) among smokers in primary care.
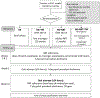
Methods: In this single-center pilot randomized trial, eligible smokers in primary care are offered brief advice by phone and randomly assigned to one of four interventions: (1) Brief advice only, (2) text messages targeted to primary care patients and tailored to quit readiness, (3) a 2-week supply of nicotine patches and/or lozenges (NRT), and (4) both text messaging and NRT. Randomization is stratified by practice and intention to quit. The text messages (up to 5/day) encourage those not ready to quit to practice a quit attempt, assist those with a quit date through a quit attempt, and promote NRT use. The 2-week supply of NRT is mailed to patients' homes.
Results: Feasibility outcomes include recruitment rates, study retention, and treatment adherence. Clinical outcomes are assessed at 1, 2, 6, and 12-weeks post-enrollment. The primary outcome is ≥1self-reported quit attempt(s). Secondary clinical outcomes include self-reported past 7- and 30-day abstinence, days not smoked, NRT adherence, and exhaled carbon monoxide.
Conclusions: This pilot assesses text messaging plus NRT, as a proactively offered intervention for smoking cessation support in smokers receiving primary care and will inform full-scale randomized trial planning.
Trial registration: ClinicalTrials.govNCT03174158.
Keywords: Mobile health; Nicotine replacement therapy; Primary care; Smoking cessation; Text messaging.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
References
-
- Centers for Disease Control and Prevention. Cigarette smoking among adults--United States, 2000. MMWRMorbidity and mortality weekly report. 2002;51(29):642–645. - PubMed
-
- Centers for Disease Control and Prevention. Quitting smoking among adults--United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–1519. - PubMed
-
- National Cancer Institute. QuitNowTXT Message Library. 2011.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous