Bortezomib-based induction followed by stem cell transplantation in light chain amyloidosis: results of the multicenter HOVON 104 trial
- PMID: 30923094
- PMCID: PMC6821610
- DOI: 10.3324/haematol.2018.213900
Bortezomib-based induction followed by stem cell transplantation in light chain amyloidosis: results of the multicenter HOVON 104 trial
Abstract
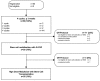
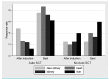
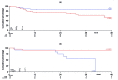
This prospective, multicenter, phase II study investigated the use of four cycles of bortezomib-dexamethasone induction treatment, followed by high-dose melphalan and autologous stem cell transplantation (SCT) in patients with newly diagnosed light chain amyloidosis. The aim of the study was to improve the hematologic complete remission (CR) rate 6 months after SCT from 30% to 50%. Fifty patients were enrolled and 72% had two or more organs involved. The overall hematologic response rate after induction treatment was 80% including 20% CR and 38% very good partial remissions (VGPR). Fifteen patients did not proceed to SCT for various reasons but mostly treatment-related toxicity and disease-related organ damage and death (2 patients). Thirty-one patients received melphalan 200 mg/m2 and four patients a reduced dose because of renal function impairment. There were no deaths related to the transplantation procedure. Hematologic responses improved at 6 months after SCT to 86% with 46% CR and 26% VGPR. However, due to the high treatment discontinuation rate before transplantation the primary endpoint of the study was not met and the CR rate in the intention-to-treat analysis was 32%. Organ responses continued to improve after SCT. We confirm the high efficacy of bortezomib-dexamethasone treatment in patients with AL amyloidosis. However, because of both treatment-related toxicity and disease characteristics, 30% of the patients could not proceed to SCT after induction treatment. (Trial registered at Dutch Trial Register identifier NTR3220).
Copyright© 2019 Ferrata Storti Foundation.
Figures

References
-
- Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641–2654. - PubMed
-
- Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–4549. - PubMed
-
- Gertz MA, Lacy MQ, Dispenzieri A, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. 2007;92(10):1415–1418. - PubMed
-
- Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood. 2013;121(26): 5124–5130. - PubMed
-
- Gertz MA, Lacy MQ, Dispenzieri A, et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48(4): 557–561. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

