A CRISPR/Cas9-based central processing unit to program complex logic computation in human cells
- PMID: 30923122
- PMCID: PMC6462112
- DOI: 10.1073/pnas.1821740116
A CRISPR/Cas9-based central processing unit to program complex logic computation in human cells
Abstract
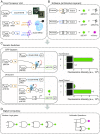
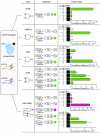
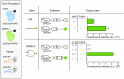
Controlling gene expression with sophisticated logic gates has been and remains one of the central aims of synthetic biology. However, conventional implementations of biocomputers use central processing units (CPUs) assembled from multiple protein-based gene switches, limiting the programming flexibility and complexity that can be achieved within single cells. Here, we introduce a CRISPR/Cas9-based core processor that enables different sets of user-defined guide RNA inputs to program a single transcriptional regulator (dCas9-KRAB) to perform a wide range of bitwise computations, from simple Boolean logic gates to arithmetic operations such as the half adder. Furthermore, we built a dual-core CPU combining two orthogonal core processors in a single cell. In principle, human cells integrating multiple orthogonal CRISPR/Cas9-based core processors could offer enormous computational capacity.
Keywords: biocomputing; genetic engineering; synthetic biology.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Xie M, Fussenegger M. Mammalian designer cells: Engineering principles and biomedical applications. Biotechnol J. 2015;10:1005–1018. - PubMed
-
- Bonnet J, Yin P, Ortiz ME, Subsoontorn P, Endy D. Amplifying genetic logic gates. Science. 2013;340:599–603. - PubMed
-
- Gaber R, et al. Designable DNA-binding domains enable construction of logic circuits in mammalian cells. Nat Chem Biol. 2014;10:203–208. - PubMed
-
- Ausländer S, Ausländer D, Müller M, Wieland M, Fussenegger M. Programmable single-cell mammalian biocomputers. Nature. 2012;487:123–127. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

