Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension
- PMID: 30923187
- PMCID: PMC6551213
- DOI: 10.1183/13993003.02004-2018
Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension
Abstract
Background: Current pulmonary hypertension treatment guidelines recommend use of a risk stratification model encompassing a range of parameters, allowing patients to be categorised as low, intermediate or high risk. Three abbreviated versions of this risk stratification model were previously evaluated in patients with pulmonary arterial hypertension (PAH) in the French, Swedish and COMPERA registries. Our objective was to investigate the three abbreviated risk stratification methods for patients with mostly prevalent PAH and chronic thromboembolic pulmonary hypertension (CTEPH), in patients from the PATENT-1/2 and CHEST-1/2 studies of riociguat.
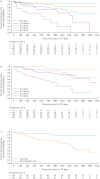
Methods: Risk was assessed at baseline and at follow-up in PATENT-1 and CHEST-1. Survival and clinical worsening-free survival were assessed in patients in each risk group/strata.
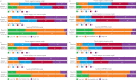
Results: With all three methods, riociguat improved risk group/strata in patients with PAH after 12 weeks. The French non-invasive and Swedish/COMPERA methods discriminated prognosis for survival and clinical worsening-free survival at both baseline and follow-up. Furthermore, patients achieving one or more low-risk criteria or a low-risk stratum at follow-up had a significantly reduced risk of death and clinical worsening compared with patients achieving no low-risk criteria or an intermediate-risk stratum. Similar results were obtained in patients with inoperable or persistent/recurrent CTEPH.
Conclusions: This analysis confirms and extends the results of the registry analyses, supporting the value of goal-oriented treatment in PAH. Further assessment of these methods in patients with CTEPH is warranted.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: H.W. Farber reports grants from Actelion, Gilead and United Therapeutics, personal fees from Actelion, Bayer AG, Bellerophon, Gilead and United Therapeutics, during the conduct of the study. Conflict of interest: H-A. Ghofrani reports grants and personal fees from Actelion, Bayer AG, Ergonex and Pfizer, personal fees from Gilead, GSK, Merck and Novartis, outside the submitted work. Conflict of interest: R.L. Benza reports grants from Bellerophon, Bayer AG, Actelion and EIGER, during the conduct of the study. Conflict of interest: D. Busse was an external employee of Bayer AG, during the conduct of the study. Conflict of interest: C. Meier was an employee of Bayer AG, during the conduct of the study. Conflict of interest: M.M. Hoeper reports consultancy fees from Actelion, Bayer AG, GSK and Pfizer, during the conduct of the study. Conflict of interest: M. Humbert reports personal fees from Bayer and Merck, during the conduct of the study; personal fees from Actelion, and support from GSK, Johnson and Johnson and United Therapeutics, outside the submitted work.
Figures





References
-
- Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. - PubMed
-
- McLaughlin VV, Archer SL, Badesch DB, et al. . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. - PubMed
-
- Ghofrani HA, Humbert M, Langleben D, et al. . Riociguat: mode of action and clinical development in pulmonary hypertension. Chest 2017; 151: 468–480. - PubMed
-
- Kim NH, Delcroix M, Jenkins DP, et al. . Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: 25 Suppl., D92–D99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
