The emerging role of cell-free DNA as a molecular marker for cancer management
- PMID: 30923679
- PMCID: PMC6425120
- DOI: 10.1016/j.bdq.2019.100087
The emerging role of cell-free DNA as a molecular marker for cancer management
Abstract
An increasing number of studies demonstrate the potential use of cell-free DNA (cfDNA) as a surrogate marker for multiple indications in cancer, including diagnosis, prognosis, and monitoring. However, harnessing the full potential of cfDNA requires (i) the optimization and standardization of preanalytical steps, (ii) refinement of current analysis strategies, and, perhaps most importantly, (iii) significant improvements in our understanding of its origin, physical properties, and dynamics in circulation. The latter knowledge is crucial for interpreting the associations between changes in the baseline characteristics of cfDNA and the clinical manifestations of cancer. In this review we explore recent advancements and highlight the current gaps in our knowledge concerning each point of contact between cfDNA analysis and the different stages of cancer management.
Keywords: Biomarker; Cancer; Cell-free DNA; Circulating tumor DNA; Liquid biopsy; Precision oncology.
Figures



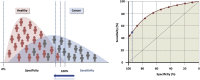
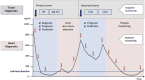

References
-
- Mandel P. Les acides nucleiques du plasma sanguin chez 1 homme. CR Seances Soc. Biol. Fil. 1948;142:241–243. - PubMed
-
- Fleischhacker M., Schmidt B. Circulating nucleic acids (CNAs) and cancer—a survey. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2007;1775:181–232. - PubMed
-
- Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46:318–322. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
