Mechanical and Biological Advantages of a Tri-Oval Implant Design
- PMID: 30925746
- PMCID: PMC6517945
- DOI: 10.3390/jcm8040427
Mechanical and Biological Advantages of a Tri-Oval Implant Design
Abstract
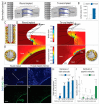
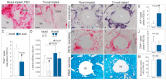
Of all geometric shapes, a tri-oval one may be the strongest because of its capacity to bear large loads with neither rotation nor deformation. Here, we modified the external shape of a dental implant from circular to tri-oval, aiming to create a combination of high strain and low strain peri-implant environment that would ensure both primary implant stability and rapid osseointegration, respectively. Using in vivo mouse models, we tested the effects of this geometric alteration on implant survival and osseointegration over time. The maxima regions of tri-oval implants provided superior primary stability without increasing insertion torque. The minima regions of tri-oval implants presented low compressive strain and significantly less osteocyte apoptosis, which led to minimal bone resorption compared to the round implants. The rate of new bone accrual was also faster around the tri-oval implants. We further subjected both round and tri-oval implants to occlusal loading immediately after placement. In contrast to the round implants that exhibited a significant dip in stability that eventually led to their failure, the tri-oval implants maintained their stability throughout the osseointegration period. Collectively, these multiscale biomechanical analyses demonstrated the superior in vivo performance of the tri-oval implant design.
Keywords: alveolar bone remodeling/regeneration; biomechanics; bone biology; dental implant; finite element analysis (FEA); osseointegration.
Conflict of interest statement
The authors declare no conflict of interest. J.B.B. and J.A.H. are paid consultants for Nobel Biocare. All other authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


References
-
- Wennerberg A., Albrektsson T., Chrcanovic B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018;11(Suppl. 1):S123–S136. - PubMed
-
- Cardoso M.V., de Rycker J., Chaudhari A., Coutinho E., Yoshida Y., Van Meerbeek B., Mesquita M.F., da Silva W.J., Yoshihara K., Vandamme K., et al. Titanium implant functionalization with phosphate-containing polymers may favour in vivo osseointegration. J. Clin. Periodontol. 2017;44:950–960. doi: 10.1111/jcpe.12736. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

