Patient-Reported Outcome Measures for Adult Dental Patients: A Systematic Review
- PMID: 30926102
- PMCID: PMC6442935
- DOI: 10.1016/j.jebdp.2018.10.005
Patient-Reported Outcome Measures for Adult Dental Patients: A Systematic Review
Abstract
Objectives: Patient-reported outcomes (PROs) are used beside disease-oriented outcomes (eg, number of teeth, clinical attachment level) to better capture the impact of diseases or interventions. To assess PROs for dental patients (dPROs), dental PRO measures (dPROMs) are applied. The aim of this systematic review was to identify generic dPROMs for adult patients and the dPROs.
Methods: This systematic review searched the MEDLINE, Embase, and PsycINFO databases along with hand searching, through December 2017, to identify English-language, multi-item dPROMs that are oral health generic, that is, they are applicable to a broad range of adult patients.
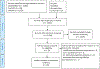
Results: We identified 20 questionnaires that contained 36 unique dPROs. They were measured by 53 dPROMs. dPRO names (N = 36) suggested they could be grouped into four dPRO categories: (1) Oral Function (N = 11), Orofacial Pain (N = 7), Orofacial Appearance (N = 3), and Psychosocial Impact (N = 14), as well as an additional dPRO that represented perceived oral health in general. Only eight questionnaires had a specific recall or reference period. dPROM's score dimensionality was only investigated in 13 of the 20 questionnaires.
Conclusions: The identified 36 dPROs represent the major aspects of an adult dental patient's oral health experience; however, four major dPRO categories, that is, Oral Function, Orofacial Pain, Orofacial Appearance, and Psychosocial Impact, summarize how patients are impacted. If multi-item, oral health-generic dPROMs are to be used to measure patients' suffering, the 53 dPROMs represent current available tools. Limitations of the majority of these dPROMs include incomplete knowledge about their dimensionality, which affects their validity, and an unspecified recall period, which reduces their clinical applicability.
Keywords: Dimensionality; Oral health; Patient-reported outcome; Patient-reported outcome measure; Quality of life; Questionnaire.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interest:
None.
Figures

References
-
- Gwaltney CJ. Patient-reported outcomes (PROs) in dental clinical trials and product development: introduction to scientific and regulatory considerations. J Evid Based Dent Pract. 2010;10(2):86–90. - PubMed
-
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. - PMC - PubMed
-
- Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3–11. - PubMed
-
- Atchison KA, Dolan TA. Development of the Geriatric Oral Health Assessment Index. J Dent Educ. 1990;54(11):680–687. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

