Identification, characterization, and structural analyses of a fungal endo-β-1,2-glucanase reveal a new glycoside hydrolase family
- PMID: 30926603
- PMCID: PMC6514616
- DOI: 10.1074/jbc.RA118.007087
Identification, characterization, and structural analyses of a fungal endo-β-1,2-glucanase reveal a new glycoside hydrolase family
Abstract
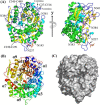
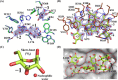
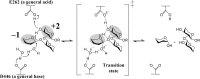
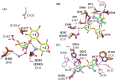
endo-β-1,2-Glucanase (SGL) is an enzyme that hydrolyzes β-1,2-glucans, which play important physiological roles in some bacteria as a cyclic form. To date, no eukaryotic SGL has been identified. We purified an SGL from Talaromyces funiculosus (TfSGL), a soil fungus, to homogeneity and then cloned the complementary DNA encoding the enzyme. TfSGL shows no significant sequence similarity to any known glycoside hydrolase (GH) families, but shows significant similarity to certain eukaryotic proteins with unknown functions. The recombinant TfSGL (TfSGLr) specifically hydrolyzed linear and cyclic β-1,2-glucans to sophorose (Glc-β-1,2-Glc) as a main product. TfSGLr hydrolyzed reducing-end-modified β-1,2-gluco-oligosaccharides to release a sophoroside with the modified moiety. These results indicate that TfSGL is an endo-type enzyme that preferably releases sophorose from the reducing end of substrates. Stereochemical analysis demonstrated that TfSGL is an inverting enzyme. The overall structure of TfSGLr includes an (α/α)6 toroid fold. The substrate-binding mode was revealed by the structure of a Michaelis complex of an inactive TfSGLr mutant with a β-1,2-glucoheptasaccharide. Mutational analysis and action pattern analysis of β-1,2-gluco-oligosaccharide derivatives revealed an unprecedented catalytic mechanism for substrate hydrolysis. Glu-262 (general acid) indirectly protonates the anomeric oxygen at subsite -1 via the 3-hydroxy group of the Glc moiety at subsite +2, and Asp-446 (general base) activates the nucleophilic water via another water. TfSGLr is apparently different from a GH144 SGL in the reaction and substrate recognition mechanism based on structural comparison. Overall, we propose that TfSGL and closely-related enzymes can be classified into a new family, GH162.
Keywords: Talaromyces funiculosus; endo-β-1,2-glucanase; enzyme catalysis; enzyme structure; fungi; glycoside hydrolase; novel glycoside hydrolase family; oligosaccharide; β-1,2-glucan; β-1,2-gluco-oligosaccharide.
© 2019 Tanaka et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures












References
-
- Ndeh D., Rogowski A., Cartmell A., Luis A. S., Baslé A., Gray J., Venditto I., Briggs J., Zhang X., Labourel A., Terrapon N., Buffetto F., Nepogodiev S., Xiao Y., Field R. A., et al. (2017) Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70 10.1038/nature21725 - DOI - PMC - PubMed
-
- Dell A., York W. S., McNeil M., Darvill A. G., and Albersheim P. (1983) The cyclic structure of β-d-(1→2)-linked d-glucans secreted by Rhizobia and Agrobacteria. Carbohydr. Res. 117, 185–200 10.1016/0008-6215(83)88086-0 - DOI
-
- Koizumi K., Okada Y., Utamura T., Hisamatsu M., and Amemura A. (1984) Further studies on the separation of cyclic (1→2)-β-d-glucans (cyclosophoraoses) produced by Rhizobium meliloti IFO 13336, and determination of their degrees of polymerization by high-performance liquid chromatography. J. Chromatogr. 299, 215–224 10.1016/S0021-9673(01)97833-1 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

