Rad52 prevents excessive replication fork reversal and protects from nascent strand degradation
- PMID: 30926821
- PMCID: PMC6441034
- DOI: 10.1038/s41467-019-09196-9
Rad52 prevents excessive replication fork reversal and protects from nascent strand degradation
Erratum in
-
Author Correction: Rad52 prevents excessive replication fork reversal and protects from nascent strand degradation.Nat Commun. 2019 May 1;10(1):2023. doi: 10.1038/s41467-019-10072-9. Nat Commun. 2019. PMID: 31043602 Free PMC article.
Abstract
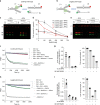
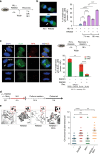
Stabilisation of stalled replication forks prevents excessive fork reversal and their pathological degradation, which can undermine genome integrity. Here we investigate a physiological role of RAD52 at stalled replication forks by using human cell models depleted of RAD52, a specific small-molecule inhibitor of the RAD52-ssDNA interaction, in vitro and single-molecule analyses. We demonstrate that RAD52 prevents excessive degradation of reversed replication forks by MRE11. Mechanistically, RAD52 binds to the stalled replication fork, promotes its occlusion and counteracts loading of SMARCAL1 in vitro and in vivo. Loss of the RAD52 function results in a slightly-defective replication restart, persistence of under-replicated regions and chromosome instability. Moreover, the RAD52-inhibited cells rely on RAD51 for completion of replication and viability upon replication arrest. Collectively, our data suggest an unexpected gatekeeper mechanism by which RAD52 limits excessive remodelling of stalled replication forks, thus indirectly assisting RAD51 and BRCA2 in protecting forks from unscheduled degradation and preventing genome instability.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

