Electrotaxis of Glioblastoma and Medulloblastoma Spheroidal Aggregates
- PMID: 30926929
- PMCID: PMC6441013
- DOI: 10.1038/s41598-019-41505-6
Electrotaxis of Glioblastoma and Medulloblastoma Spheroidal Aggregates
Abstract
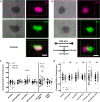
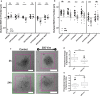
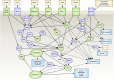
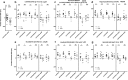
Treatment of neuroepithelial cancers remains a daunting clinical challenge, particularly due to an inability to address rampant invasion deep into eloquent regions of the brain. Given the lack of access, and the dispersed nature of brain tumor cells, we explore the possibility of electric fields inducing directed tumor cell migration. In this study we investigate the properties of populations of brain cancer undergoing electrotaxis, a phenomenon whereby cells are directed to migrate under control of an electrical field. We investigate two cell lines for glioblastoma and medulloblastoma (U87mg & DAOY, respectively), plated as spheroidal aggregates in Matrigel-filled electrotaxis channels, and report opposing electrotactic responses. To further understand electrotactic migration of tumor cells, we performed RNA-sequencing for pathway discovery to identify signaling that is differentially affected by the exposure of direct-current electrical fields. Further, using selective pharmacological inhibition assays, focused on the PI3K/mTOR/AKT signaling axis, we validate whether there is a causal relationship to electrotaxis and these mechanisms of action. We find that U87 mg electrotaxis is abolished under pharmacological inhibition of PI3Kγ, mTOR, AKT and ErbB2 signaling, whereas DAOY cell electrotaxis was not attenuated by these or other pathways evaluated.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Howlader, N., et al. NCI SEER Cancer Statistics Review, 1975-2014https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, September 2017 (2016).
-
- Wiesner SM, Freese A, Ohlfest JR. Emerging concepts in glioma biology: implications for clinical protocols and rational treatment strategies. Neurosurgical focus. 2005;19(4):1–6. - PubMed
-
- Ricard D, et al. Primary brain tumours in adults. The Lancet. 2012;379(9830):1984–1996. - PubMed
-
- Bernstein JJ, Woodard CA. Glioblastoma cells do not intravasate into blood vessels. Neurosurgery. 1995;36(1):124–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

