PIXUL-ChIP: integrated high-throughput sample preparation and analytical platform for epigenetic studies
- PMID: 30927002
- PMCID: PMC6614803
- DOI: 10.1093/nar/gkz222
PIXUL-ChIP: integrated high-throughput sample preparation and analytical platform for epigenetic studies
Abstract
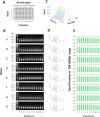
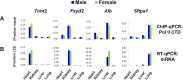
Chromatin immunoprecipitation (ChIP) is the most widely used approach for identification of genome-associated proteins and their modifications. We have previously introduced a microplate-based ChIP platform, Matrix ChIP, where the entire ChIP procedure is done on the same plate without sample transfers. Compared to conventional ChIP protocols, the Matrix ChIP assay is faster and has increased throughput. However, even with microplate ChIP assays, sample preparation and chromatin fragmentation (which is required to map genomic locations) remains a major bottleneck. We have developed a novel technology (termed 'PIXUL') utilizing an array of ultrasound transducers for simultaneous shearing of samples in standard 96-well microplates. We integrated PIXUL with Matrix ChIP ('PIXUL-ChIP'), that allows for fast, reproducible, low-cost and high-throughput sample preparation and ChIP analysis of 96 samples (cell culture or tissues) in one day. Further, we demonstrated that chromatin prepared using PIXUL can be used in an existing ChIP-seq workflow. Thus, the high-throughput capacity of PIXUL-ChIP provides the means to carry out ChIP-qPCR or ChIP-seq experiments involving dozens of samples. Given the complexity of epigenetic processes, the use of PIXUL-ChIP will advance our understanding of these processes in health and disease, as well as facilitate screening of epigenetic drugs.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures








References
-
- Orlando V., Strutt H., Paro R.. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997; 11:205–214. - PubMed
-
- O’Neill L.P., Turner B.M.. Immunoprecipitation of chromatin. Methods Enzymol. 1996; 274:189–197. - PubMed
-
- Huebert D.J., Kamal M., O’Donovan A., Bernstein B.E.. Genome-wide analysis of histone modifications by ChIP-on-chip. Methods. 2006; 40:365–369. - PubMed
-
- Heintzman N.D., Stuart R.K., Hon G., Fu Y., Ching C.W., Hawkins R.D., Barrera L.O., Van Calcar S., Qu C., Ching K.A. et al. .. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007; 39:311–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

